Abstract
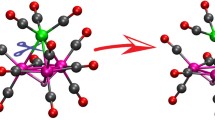
The X-ray crystal structures of [NHR3]2[Fe4S4X4] (X = PhS, R = Et or nBu; X = Cl, R = nBu), reported in this paper, show NH…X interactions between the cation and the cubanoid cluster. Comparison of the cluster dimensions in [NHR3]2[Fe4S4X4] with those reported earlier for [NR′4]2[Fe4S4X4] (R′ = Me, X = PhS; R′ = Et, X = Cl) indicates that N–H…X interactions have a negligible effect on the dimensions of the cluster. The relevance of these structures to understanding where on [Fe4S4X4]2− protonation labilises the cluster to substitution is discussed.




Similar content being viewed by others
References
Burgess B, Lowe DJ (1996) Chem Rev 96:2983
Spatzal T, Akoyoglu M, Zhang L, Andrade SLA, Schleicher E, Weber S, Rees DC, Einsle O (2011) Science 334:940
Hoffman BM, Dean DR, Seefeldt LC (2009) Acc Chem Res 42:609
Lukoyanov D, Yang Z-Y, Khadka N, Dean DR, Seefeldt LC, Hoffman BM (2015) J Am Chem Soc 137:3610
Holm RH, Kennepohl P, Solomon EI (1996) Chem Rev 96:2239
Beinert H, Kennedy MC, Stout CD (1996) Chem Rev 96:2335
Lee SC, Holm RH (2004) Chem Rev 104:1135
Dukes GR, Holm RH (1975) J Am Chem Soc 97:528
Henderson RA (2005) Chem Rev 105:2365
Henderson RA (2005) Coord Chem Rev 249:1841
Henderson RA (2012) Bioinorg React Mech 8:1
Alwaaly A, Dance I, Henderson RA (2014) Chem Commun 50:4799
Dance I, Henderson RA (2014) Dalton Trans 43:16213
Dance I (2015) Dalton Trans 44:4707
Al-Rammahi TMM, Henderson RA (2016) Dalton Trans 45:307
Al-Rammahi TMM, Henderson RA (2016) Dalton Trans 45:1373
Henderson RA, Oglieve KE (1999) J Chem Soc Dalton Trans 3927
Dunford AJ, Henderson RA (2002) J Chem Soc Dalton Trans 2837
Bell J, Dunford AJ, Hollis E, Henderson RA (2003) Angew Chem Int Ed 42:1149
Bates K, Henderson RA (2008) Inorg Chem 47:5850
Garrett B, Henderson RA (2010) Dalton Trans 39:4586
Christou G, Garner CD, Balasubramaniam A, Ridge B, Rydon HN (1982) Inorg Synth 21:33
Hagen KS, Reynolds JG, Holm RH (1981) J Am Chem Soc 103:4054
Que L Jr, Bobrik MA, Ibers JA, Holm RH (1974) J Am Chem Soc 96:4168
Bobrik MA, Hodgson KO, Holm RH (1977) Inorg Chem 16:1851
Scheiner S (1985) Acc Chem Res 18:174
Izutsu K (1990) Acid-base dissociation constants in aprotic solvents. Blackwell, Oxford
Stephens PJ, Jollie JR, Werschel A (1996) Chem Rev 96:2491 (and references therein)
Ueyama N, Yamada Y, Okamura T, Kimura S, Nakamura A (1996) Inorg Chem 35:6473
Strasdeit H, Krebs B, Henkel G (1984) Inorg Chem 23:1816
You J-F, Papaefthymiou GC, Holm RH (1992) J Am Chem Soc 114:2697
You J-F, Snyder BS, Papaefthymiou GC, Holm RH (1990) J Am Chem Soc 112:1067
Henderson RA (1999) J Chem Soc Dalton Trans 119
Dilworth JR, Henderson RA, Dahlstrom P, Nicholson T, Zubieta JA (1987) J Chem Soc Dalton Trans 529
CrysAlisPro (Version 1.171.35) (2010) Oxford diffraction
Clark RC, Reid JS (1995) Acta Cryst A51:887
Dolomanov OV, Bouhis LJ, Gildea RJ, Howard JAK, Puschmann H (2009) J Appl Cryst 42:339
Sheldrick GM (2015) Acta Cryst A71:3
Sheldrick GM (2008) Acta Cryst A64:112
Acknowledgments
T. Al-Rammahi thanks the Higher Committee for Education Development in Iraq and Iraqi Ministry of Higher Education and Scientific Research for a studentship.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Al-Rammahi, T.M.M., Waddell, P.G. & Henderson, R.A. X-ray crystal structures of [NHR3]2[Fe4S4X4] (X = PhS, R = Et or nBu; X = Cl, R = nBu): implications for sites of protonation in Fe–S clusters. Transit Met Chem 41, 555–561 (2016). https://doi.org/10.1007/s11243-016-0052-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11243-016-0052-5