Abstract
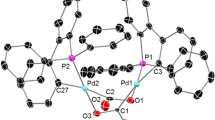
The Suzuki–Miyaura reaction of various aryl halides with aryl boronic acids using {[Ph2PCH2PPh2CH=C(O)(C10H7)]PdCl2} as a catalyst has been investigated. The X-ray crystal structure of the catalyst reveals a five-membered chelate ring formed by coordination of the ligand through the phosphine group and the ylidic carbon atom to the metal center. This palladacycle exhibited excellent activities and reusability in the aqueous phase for the Suzuki cross-coupling reactions of arylboronic acids with aryl halides. The proposed protocol featured mild reaction conditions and notable simplicity and efficiency using Cs2CO3 as a base in water. The catalytic system could be reused four times without significant loss of activity.





Similar content being viewed by others
References
Zhou Z-Z, Liu F-S, Shen D-S, Tan C, Luo L-Y (2011) Inorg Chem Commun 14:659–662
Littke AF, Fu GC (2002) Angew Chem Int Ed 41:4176–4211
Alonso F, Beletskaya IP, Yus M (2008) Tetrahedron 64:3047–3101
Polshettiwar V, Decottignies A, Len C, Fihri A (2010) ChemSusChem 3:502–522
Teo S, Weng Z, Hor T (2011) J Organomet Chem 696:2928–2934
Tang Y-Q, Lu J-M, Shao L-X (2011) J Organomet Chem 696:3741–3744
Lasri J, MacLeod TC, Pombeiro AJ (2011) Appl Catal A Gen 397:94–102
Alizadeh A, Khodaei M, Kordestani D, Beygzadeh M (2013) Tetrahedron Lett 54:291–294
Weng Z, Teo S, Hor TA (2007) Acc Chem Res 40:676–684
Fu GC (2008) Acc Chem Res 41:1555–1564
Molander GA, Canturk B (2009) Angew Chem Int Ed 48:9240–9261
Martin R, Buchwald SL (2008) Acc Chem Res 41:1461–1473
Punji B, Ganesamoorthy C, Balakrishna MS (2006) J Mol Catal A Chem 259:78–83
Bedford RB, Welch SL (2001) Chem Commun, pp 129–130
Aizawa S-I, Hase T, Wada T (2007) J Organomet Chem 692:813–818
Chahen L, Therrien B, Süss-Fink G (2006) J Organomet Chem 691:4257–4264
Milde B, Schaarschmidt D, Ecorchard P, Lang H (2012) J Organomet Chem 706:52–65
Aizawa S-I, Majumder A, Yokoyama Y, Tamai M, Maeda D, Kitamura A (2009) Organometallics 28:6067–6072
Milde B, Packheiser R, Hildebrandt S, Schaarschmidt D, Rüffer T, Lang H (2012) Organometallics 31:3661–3671
Sabounchei SJ, Panahimehr M, Ahmadi M, Akhlaghi F, Boscovic C (2014) C R Chim 17:81–90
Frey GD, Schütz J, Herdtweck E, Herrmann WA (2005) Organometallics 24:4416–4426
Miyaura N, Yanagi T, Suzuki A (1981) Synth Commun 11:513–519
Farina V (2004) Adv Synth Catal 346:1553–1582
Dupont J, Consorti CS, Spencer J (2005) Chem Rev 105:2527–2572
Subhas MS, Racharlawar SS, Sridhar B, Kennady PK, Likhar PR, Kantam ML, Bhargava SK (2010) Org Biomol Chem 8:3001–3006
Sabounchei SJ, Ahmadi M, Panahimehr M, Bagherjeri FA, Nasri Z (2014) J Mol Catal A Chem 383:249–259
Sabounchei SJ, Panahimehr M, Ahmadi M, Nasri Z, Khavasi HR (2013) J Organomet Chem 723:207–213
D. S. C. GmbH, 1.30 ed., Germany, 2005, Program for the acquisition and analysis of data
D. S. C. GmbH, Germany., 1.28b ed., 2005, Program for data reduction and absorption correction
D. S. C. GmbH, Germany., 2.05 ed., 2004, Program for crystal optimization for numerical absorption correction
Sheldrick GM (2007) Acta Crystallogr A 64:112–122
D. S. C. GmbH, Germany., 1.07b ed., 2000, Crystallographic package
Spek AL (2009) Acta Crystallogr D65:148–155
Steffen W, Palenik GJ (1976) Inorg Chem 15:2432–2439
Falvello LR, Margalejo ME, Navarro R, Urriolabeitia EP (2003) Inorg Chim Acta 347:75–85
Sabounchei SJ, Panahimehr M, Khavasi HR, Bagherjeri FA, Boscovic C (2014) Chem Pap 68:624–632
Champness NR, Kelly PF, Levason W, Reid G, Slawin AM, Williams DJ (1995) Inorg Chem 34:651–657
Batchelor RJ, Einstein FW, Gay ID, Gu J, Pinto BM, Zhou X (1996) Inorg Chem 35:3667–3674
Downard AJ, Bond AM, Clayton AJ, Hanton LR, McMorran DA (1996) Inorg Chem 35:7684–7690
Sabounchei SJ, Samiee S, Nematollahi D, Naghipour A, Morales-Morales D (2010) Inorg Chim Acta 363:3973–3980
Sabounchei SJ, Shahriary P, Salehzadeh S, Gholiee Y, Nematollahi D, Chehregani A, Amani A (2014) New J Chem 38:1199–1210
Sabounchei SJ, Shahriary P, Salehzadeh S, Gholiee Y, Nematollahi D, Chehregani A, Amani A, Afsartala Z (2014) Spectrochim Acta A 135:1019–1031
Cotton FA (1998) A comprehensive text. Intersci, p 917
Wilkinson G, Gillard R, McCleverty J (1987) Pergamon, Oxford, pp 534–774
Sabounchei SJ, Ahmadi M, Nasri Z, Shams E, Salehzadeh S, Gholiee Y, Karamian R, Asadbegy M, Samiee S (2013) C R Chim 16:159–175
Chatt J, Hart F, Watson H (1962) J Chem Soc, pp 2537–2545
Marziale AN, Jantke D, Faul SH, Reiner T, Herdtweck E, Eppinger J (2011) Green Chem 13:169–177
Acknowledgments
We are grateful to Bu-Ali Sina University for financial support.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Sabounchei, S.J., Hosseinzadeh, M., Panahimehr, M. et al. A palladium–phosphine catalytic system as an active and recycable precatalyst for Suzuki coupling in water. Transition Met Chem 40, 657–663 (2015). https://doi.org/10.1007/s11243-015-9959-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11243-015-9959-5