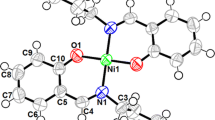
Abstract
The synthesis, crystal structure and electrochemical properties of a Ni(II) Schiff base complex, [Ni(L)]PF6 (where L is 2,4,9,11,11-pentamethyl-2,3,4 triaza-1-one-4-amine) are reported herein. The complex has been characterized by its electrochemical behavior, X-ray crystallographic structural analysis, physio-chemical methods and spectroscopic techniques. Electrospray mass spectroscopic analysis gives a dominant ion peak with m/z = 296 which corresponds to the {[Ni(L)]PF6–HPF6}+ fragment. Cyclic voltammograms for [Ni(L)]PF6, obtained in DMF (0.1 M Bu4NPF6) at a glassy carbon electrode with a scan rate of 100 mV s−1, exhibit reversible ([NiII(L)]+/[NiI(L)]) reduction and chemically irreversible ([NiII(L)]+/[NiIII(L)]2+→ electroactive product) oxidation processes at −2.05 and 0.62 V, respectively. The diffusion coefficient, calculated using the Randles–Sevcik relationship, is 9.7 × 10−6 cm2 s−1. Electrochemical studies reveal that the NiI reduced form of the complex is capable of catalyzing CO2 reduction at a potential that is thermodynamically more favorable than for the reduced [Ni(N,N′-ethylenebis(acetylacetoneiminato)]complex. Spectroelectrochemical analyses following bulk electrolysis of [Ni(L)]PF6 under CO2 revealed the formation of oxalate and bicarbonate.






Similar content being viewed by others
References
Curtis NF (1968) Coord Chem Rev 3:23
Fisher BJ, Eisenberg R (1980) J Am Chem Soc 102:7361–7363
Fujita E, Szalda DJ, Creutz C, Sutin N (1988) J Am Chem Soc 110:4870–4871
Fujita E, Creutz C, Sutin N, Szalda DJ (1991) J Am Chem Soc 113:343–353
Ray A, Seth BK, Pal U, Basu S (2012) Spectrochim Acta, Part A 92:164–174
Gupta SK, Hitchcock PB, Kushwah YS (2002) J Coord Chem 55:1401–1407
Fabbrizzi L, Lari A, Poggi A, Seghi B (1982) Inorg Chem 21:2083–2085
Nias MS, Adaryani RIMA, Heydarzadeh S (2005) Transit Metal Chem 30:445–450
Udugala-Ganehenege MY, Heeg MJ, Hryhorczuk LM, Wenger LE, Endicott JF (2001) Inorg Chem 40:1614–1625
CrysAlisPro (2010) v 1.171.34.36, Oxford Diffraction Ltd (Agilent Technologies), Oxfordshire UK
Sheldrick GM (2008) Acta Cryst Sect A 64:112–122
Schwekendiek K, Glorius F (2006) Synthesis 2996–3002. http://www.organic-chemistry.org/synthesis/heterocycles/2-oxazolines.shtm. Accessed 17 May 2014
Udugala-Ganehenege MY et al. (2014) Published online on 02 Aug 2014 in Transition metal Chemistry. doi:10.1007/s11243-014-9864-3
Suh MP (1997) Adv Inorg Chem 44:93–146
Simandi L (1992) Catalytic activation of dioxygen by metal complexes. Kluwer Academic Publishers, Dordrecht, pp 318–331
Scibioh MA, Ragini PV, Rani S, Vijayaraghavan VR, Viswanathan B (2001) Indian Academy of Sciences. Proc Indian Acad Sci (Chem. Sci.) 113(4):343–350
Balazs GB, Anson FC (1992) J Electroanal Chem 322:325–345
Balazs GB, Anson FC (1993) J Electroanal Chem 361:149–157
Alwis C, Crayston JA, Cromie T, Eisenblatter T, Hay RW, Lampeka YD, Tsymbal LV (2000) Electrochim Acta 45:2061–2074
Grochala W (2006) Phys Chem Chem Phys 8:1340–1345
Cheng SC, Blaine CA, Hill MG, Mann KR (1996) Inorg Chem 35:7704–7708
Acknowledgments
The authors gratefully acknowledge financial assistance from the Australian Research Council and an Endeavour Award-2011 Post-doctoral Fellowship by DEEWR and AusAid of Australia to Dr. (Mrs.) M.Y.Udugala-Ganehenege of University of Peradeniya, Sri Lanka. Assistance given by Ms. S.S. Hettiarachchi and Ms. M.C.R Peiris in carrying out the bulk electrolysis and FTIR measurements also is highly appreciated.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Udugala-Ganehenege, M.Y., Liu, Y., Forsyth, C. et al. Synthesis, characterization, crystal structure, electrochemical properties and electrocatalytic activity of an unexpected nickel(II) Schiff base complex derived from bis(acetylacetonato)nickel(II), acetone and ethylenediamine. Transition Met Chem 39, 883–891 (2014). https://doi.org/10.1007/s11243-014-9872-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11243-014-9872-3