Abstract
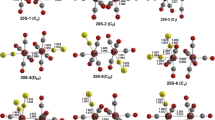
The iron tricarbonyl complex of octafluorocyclooctatetraene was synthesized by Hughes and co-workers and shown by X-ray crystallography to have a trihapto–monohapto structure (η3,1-C8F8)Fe(CO)3 in contrast to the tetrahapto structure (η4-C8H8)Fe(CO)3 formed by the non-fluorinated cyclooctatetraene. This difference has stimulated a comprehensive density functional theoretical study of the octafluorocyclooctatetraene metal carbonyl complexes (C8F8)M(CO) n (n = 4, 3, 2, 1 for M = Ti, V, Cr, Mn, and Fe; n = 3, 2, 1 for M = Co, Ni) for comparison with their hydrogen analogues (C8H8)M(CO) n . In most such systems, the substitution of fluorine for hydrogen leads to relatively small changes in the preferred structures. However, for the iron carbonyl derivatives (C8X8)Fe(CO)3 (X = H, F), the difference observed experimentally has been confirmed by theory with (η3,1-C8F8)Fe(CO)3 and (η4-C8H8)Fe(CO)3 being the lowest energy structures by 4 and 14 kcal/mol, respectively. The ligand exchange reactions C8H8 + (C8F8)M(CO) n → C8F8 + (C8H8)M(CO) n are predicted to be exothermic for almost all of the systems considered, with the (η3,1-C8X8)Fe(CO)3 system being the main exception. This suggests that the C8F8 ligand generally bonds more weakly to transition metals than the C8H8 ligand in accord with the electron-withdrawing effect of the ligand fluorine atoms.













Similar content being viewed by others
References
Fray GI, Saxton RG (1978) The chemistry of cyclooctatetraene and its derivatives. Cambridge University Press, Cambridge
Barefoot A C III, Corcoran E W Jr, Hughes RP, Lemal DM, Saunders WD, Laird BB, Davis RE (1981) J Am Chem Soc 103:970
Deganello G (1979) Transition metal complexes of cyclic polyolefins. Academic Press, New York
Manuel TA, Stone FGA (1959) Proc Chem Soc Lond 90
Manuel TA, Stone FGA (1960) J Am Chem Soc 82:366
Rausch MD, Schrauzer GN (1959) Chem Ind 957
Nakamura A, Hagihara N (1959) Bull Chem Soc Jpn 32:880
Stone FGA (1972) Pure Appl Chem 30:551
Hughes RP (1990) Adv Organometal Chem 31:183
Hughes RP (2010) J Fluor Chem 131:1059
Lemal DM, Buzby JM, Barefoot III AC, Grayston MW, Laganis ED (1980) J Org Chem 45:3118
Hughes RP, Samkoff DE, Davis RE, Laird BB (1983) Organometallics 2:195
Hemond RC, Hughes RP, Rheingold AL (1989) Organometallics 8:1261
Kreiter CG, Maasbol A, Anet FAL, Kaesz HD, Winstein S (1966) J Am Chem Soc 88:3444
King RB (1967) J Organometal Chem 8:139
Wang H, Du Q, Xie Y, King RB, Schaefer HF (2010) J Organometal Chem 695:215
Ziegler T, Autschbach J (2005) Chem Rev 105:2695
Bühl M, Kabrede H (2006) J Chem Theory Comput 2:1282
Brynda M, Gagliardi L, Widmark PO, Power PP, Roos BO (2006) Angew Chem Int Ed 45:3804
Sieffert N, Bühl M (2010) J Am Chem Soc 132:8056
Schyman P, Lai W, Chen H, Wang Y, Shaik S (2011) J Am Chem Soc 133:7977
Adams RD, Pearl WC, Wong YO, Zhang Q, Hall MB, Walensky JR (2011) J Am Chem Soc 133:12994
Lonsdale R, Olah J, Mulholland AJ, Harvey JN (2011) J Am Chem Soc 133:15464
Becke AD (1993) J Chem Phys 98:5648
Lee C, Yang W, Parr RG (1988) Phys Rev B 37:785
Becke AD (1988) Phys Rev A 38:3098
Perdew JP (1986) Phys Rev B 33:8822
Furche F, Perdew JP (2006) J Chem Phys 124:044103
Wang H, Xie Y, King RB, Schaefer HF (2005) J Am Chem Soc 127:11646
Wang H, Xie Y, King RB, Schaefer HF (2006) J Am Chem Soc 128:11376
Dunning TH (1970) J Chem Phys 53:2823
Huzinaga S (1965) J Chem Phys 42:1293
Wachters AJH (1970) J Chem Phys 52:1033
Hood DM, Pitzer RM, Schaefer HF (1979) J Chem Phys 71:705
Frisch MJ et al (2009) G09. Gaussian, Inc., Wallingford, CT
Fischer EO, Jira R (1954) Z Naturforsch 9b:618
Piper TS, Cotton FA, Wilkinson G (1955) J Inorg Nucl Chem 1:165
Reiher M, Salomon O, Hess BA (2001) Theor Chem Acc 107:48
Salomon O, Reiher M, Hess BA (2002) J Chem Phys 117:4729
Wang H, Li R, King RB (2013) J Fluor Chem 153:121
Acknowledgments
We are grateful for financial support from the China Scholarship Council and hospitality of Center for Computational Quantum Chemistry of the University of Georgia, USA. We acknowledge financial support from the Fundamental Research Funds for the Central Universities (Grant SWJTU12CX084), the China National Science Foundation (Grant 11174237), the Sichuan Province, Applied Science and Technology Project (Grant 2013JY0035), the open research fund of the Key Laboratory of Advanced Scientific Computation, Xihua University (Grant: szjj2012-035), and the US National Science Foundation (Grant CHE-1057466).
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Wang, H., Wang, H., Die, D. et al. The hapticity of octafluorocyclooctatetraene in its first-row mononuclear transition metal carbonyl complexes: effect of perfluorination. Transition Met Chem 39, 95–109 (2014). https://doi.org/10.1007/s11243-013-9778-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11243-013-9778-5