Abstract
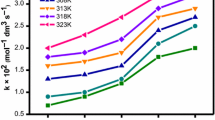
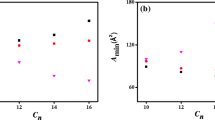
A surfactant–cobalt(III) complex, cis-[Co(en)2(4AMP)(DA)](ClO4)3, (en = ethylenediamine, 4AMP = 4-aminopyridine, DA = dodecylamine), was synthesized and characterized by physicochemical and spectroscopic methods. The critical micelle concentration (CMC) value of this surfactant–cobalt(III) complex in aqueous solution was obtained from conductance measurements. Conductivity data were used for evaluation of the temperature-dependent CMC and the thermodynamics of micellization (\( \Updelta {\text{G}}_{\text{m}}^{ 0} \), \( \Updelta {\text{H}}_{\text{m}}^{0} \), and \( \Updelta {\text{S}}_{\text{m}}^{0} \)). The kinetics of reduction of this surfactant–cobalt(III) complex by ion(II) in micelles, β-cyclodextrin (β-CD), and ionic liquid (IL) were studied. The reaction was found to be second order, and the electron transfer is postulated as outer sphere. The second-order rate constant for the electron transfer reaction was found to increase with increasing concentration of IL, but inclusion of the long aliphatic chain of the surfactant complex into β-CD decreases the rate of the reaction. The results have been interpreted in terms of the amphiphilicity of the surfactant complex.






Similar content being viewed by others
References
Tadros TF (2005) Applied surfactants, 1st edn. Wiley, Germany
Gaidamauskas E, Cleaver DP, Chatterjee PB, Crans DC (2010) Effect of micellar interface on solute location: 2,6-pyridinedicarboxylate in CTAB micelles and CTAB and AOT reverse micelles. Langmuir 26(16):13153–13161
Ghirlanda G, Scrimin P, Tecilla P, Toffoletti A (1998) Amphiphilic copper(II) complexes modeled after the metal-complexation subunit of bleomysin-antibiotics. Langmuir 14:1646–1655
Tavernier HL, Barzykin AV, Tachiya M, Fayer MD (1998) Solvent reorganization energy and free energy change for donor/acceptor electron transfer at micelle surfaces: theory and Experiment. J Phys Chem B 102:6078–6088
Hammarstro¨m L, Norrby T, Stenhagen G, Martensson J, Akermark B, Almgren MJ (1997) Two-dimensional emission quenching and charge separation using a Ru(II)-photosensitizer assembled with membrane bound acceptors. Phys Chem B 101:7494–7504
Srinivasan S, Annaraj J, Athappan PR (2005) Spectral and redox studies on mixed ligand complexes of cobalt(III) phenanthroline/bipyridyl and benzoylhydrazones, their DNA binding and antimicrobial activity. J Inorg Biochem 99:876–882
Cameron PJ, Peter LM, Zakeeruddin SM, Gratzel M (2004) Electrochemical studies of the Co(III)/Co(II)(DBBIP)2 redox couple as a mediator for dye-sensitized nanocrystalline solar cells. Coord Chem Rev 248:1447–1453
Majumdar T, Mahapatra A (2007) Kinetics of electron transfer reaction in micellar and reverse micellar media reduction of [Co(NH3)5N3]Cl2 by ion(II). Colloids Surf A 302:360–365
Sasikala K, Arunachalam S (2010) Studies on outer sphere electron transfer reactions of some surfactant-cobalt(III) complexes with ferrocyanide anion. Colloid J 72:530–537
Sasikala K, Arunachalam S (2010) Synthesis, characterization and electron transfer reactions of some surfactant- cobalt(III) complex ions. Monatsh Chemie 141:309–314
Sasikala K, Arunachalam S (2009) Studies on outer-sphere electron transfer between some surfactant-cobalt(III) complexes in micelles as well as in β-cyclodextrin. Colloids Surf A physicochem Eng Aspects 335:98–102
Santhakumar K, Kumaraguru N, Arunachalam S, Arumugam MN (2006) Trans Met Chem 31:62–70
Kumaraguru N, Arunachalam S, Arumugam MN, Santhakumar K (2006) Trans Met Chem 31:250–255
Brown P, Butts CP, Eastoe J, Fermin D, Grillo I, Lee HC, Parker D, Plana D, Richardson RM (2012) Anionic surfactant ionic liquids with 1-butyl-3-methyl-imidazolium cations: characterization and application. Langmuir 28:2502–2509
Peng L, Zhang J, Li J, Han B, Xue Z, Yang G (2012) Surfactant-directed assembly of mesoporous metal–organic framework nanoplates in ionic liquids. Chem Commun 48:8688–8690
Heintz A (2005) Recent developments in thermodynamics and thermophysics of non-aqueous mixtures containing ionic liquids. A review. J Chem Thermodyn 37:525–535
Zhang ZC (2006) Catalysis in ionic liquids. Adv catal 49:153–237
Dupont R, Souza F, Suarez PAZ (2002) Ionic liquid (molten salt) phase organometallic catalysis. Chem Rev 102:3667–3692
Comelles F, Ribosa I, González JJ, Garcia MT (2012) Interaction of nonionic surfactants and hydrophilic ionic liquids in aqueous solutions: can short ionic liquids be more than a solvent? Langmuir 28:14522–14530
Hea Y, Shanga Y, Liua Z, Shaob S, Liua H, Hua Y (2013) Interactions between ionic liquid surfactant [C12mim]Br and DNA in dilute brine. Colloids Surf B 101:398–404
Hak SC, Tooru O, Shintrao S, Nobukiko Y (2003) Control of rapid phase transition induced by supramolecular complexation of β-cyclodextrin-conjugated poly(€-lysine) with a specific guest. Macromolecules 36:5342–5347
Kitson RE (1950) Simultaneous spectrophotometric determination of cobalt, copper, and iron. Analyt Chem 22:664–667
Ghosh S, Barve AC, Kumbhar AA, Kumbhar AS, Puranik VG, Datar PA, Sonawane UB, Joshi RR (2006) Synthesis, characterization, X-ray structure and DNA photocleavage by cis-dichlorobis(diimine)Co(III) complexes. J Inorg Biochem 100:331–343
Kumaraguru N, Santhakumar K, Arunachalam S, Arumugham MN (2006) Synthesis, characterization and micellization behaviour of some surface active mixed-ligand complexes of cobalt(III). Polyhedron 25:3253–3260
Dayalan A, Revathi C (2006) Kinetics of the reduction of 4-amino and 4-cyanopyridinechlorocobaloximes by ion(II). J Serb Chem Soc 71:1311–1321
Cannon RD, Gardiner JJ (1972) Kinetics of electron transfer: the reaction of acetatopenta-amminecobalt(III) with N-methyliminodiacetatoiron(II). J Chem Soc, Dalton Trans 89:887–890
Baldwin ME (1960) The infrared spectra of cobalt(III) ethylenediamine complexes. Part 1. Vibrations of the ethylenediamine chelate ring. J Chem Soc 2:4369–4378
Buckingam DA, Jones D (1965) Infrared spectra of cobalt(III) triethylenetetramine complexes. Inorg Chem 4:1387–1392
Kipp EB, Haines RA (1969) Infrared studies of cis- and trans-bis(halogenoacetato)bis-(ethylenediamine)cobalt(III) complexes. Can J Chem 47:1073–1075
Oulaghan BM, House DA (1978) Anionopentaaminecobalt(III) complexes with polyamine ligands. Synthesis, characterization, and reaction kinetics of some cis-chlorobis(1,3-diaminopropane)(alkylamine)cobalt(III) complexes. Inorg Chem 17:2197–2202
Mukerjee P (1962) The thermodynamics of micelle formation in association colloids. J Phys Chem 66:1375–1376
Zana R (1980) Ionization of cationic micelles: effect of the detergent structure. J Colloid Interface Sci 78:330–337
Nusselder JJH, Engberts JBFN (1992) Toward a better understanding of the driving force for micelle formation and micellar growth. J Colloid Interface Sci 148:353–361
Miralles AJ, Szecsy AP, Haim A (1982) Electron transfer reactions of ion pairs: reductions of various substituted(pyridine)pentaaminecobalt(III) complexes by hexacyanoferrate(II). Inorg Chem 21:697–699
Nagaraj K, Arunachalam S (2013) Synthesis, CMC determination, and outer sphere electron transfer reaction of the surfactant complex ion, cis-[co(en)2(4CNP)(DA)]3+ with [Fe(CN)6]4− in micelles, β-cyclodextrin and liposome (dipalmidoylphosphotidylcholine) vesicles. Aust J Chem (manuscript under minor revision)
Van-etten RL, Sebastian JF, Clowes GA, Bender ML (1967) Acceleration of phenyl ester cleavage by cycloamyloses. A model for enzymatic specificity. J Am Chem Soc 89:3242–3253
Bender M, Komiyama L (1977) Cyclodextrin chemistry. Springer, New York
Karunakaran C, Chidambaranathan V (2001) Linear free energy relationships near isokinetic temperature. Oxidation of organic sulfides with nicotinium dichromate. Croat Chem Acta 74(1):51–59
Cramer F, Saenger W, Spatz H (1967) Inclusion compounds. The formation of inclusion compounds α-cyclodextrin in aqueous solutions. Thermodynamics and kinetics. J Am Chem Soc 89:14–20
Arulsamy N, Bohle DS, Goodson PA, Jaeger DA, Reddy VB (2001) Inorg Chem 40:836
Acknowledgments
We are grateful to the UGC-COSIST and DST-FIST programmes of the Department of Chemistry, Bharathidasan University, and UGC-RFSMS fellowship to one of the authors, K. Nagaraj, by Bharathidasan University. Financial assistance from the CSIR (Grant no. 01(2461)/11/EMR-II), DST (Grant No. SR/S1/IC-13/2009), and UGC (Grant no. 41-223/2012(SR) sanctioned to S. Arunachalam is also gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Nagaraj, K., Arunachalam, S. Synthesis and electron transfer kinetics of a surfactant–cobalt(III) complex: effects of micelles, β-cyclodextrin, and ionic liquids. Transition Met Chem 38, 649–657 (2013). https://doi.org/10.1007/s11243-013-9733-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11243-013-9733-5