Abstract
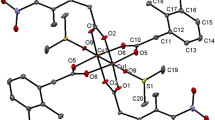
A series of thiocarboxylato and selenocarboxylato monomeric CpFe(CO)2ECORCOCl and dimeric [CpFe(CO)2ECO]2R iron complexes have been synthesized and characterized. The interaction of (μ-Ex)[CpFe(CO)2]2 (E = S; x = 2–4. E = Se; x = 1) with di-acid chlorides (ClCORCOCl) in a 1:1 molar ratio gave the monomeric complexes CpFe(CO)2ECORCOCl for R = 1,3-C6H4, 2,6-C5H3N, 1,2-C6H4. However, the dimeric complexes [CpFe(CO)2ECO]2R were obtained from the same reactants in a 2:1 metal-to-ligand molar ratio in which R is 1,3-C6H4, 2,6-C5H3N or C2H4. The monomer versus dimer production mainly depends on the electronic and steric factors of the R-moiety. The new monomeric and dimeric thio- and selenocarboxylato iron complexes have been characterized by spectroscopic techniques (1H- and 13C-NMR, IR) and by elemental analysis. The structures of [CpFe(CO)2SCO]2(1,3-C6H4) and its seleno analogue [CpFe(CO)2SeCO]2(1,3-C6H4) were determined by X-ray structure determination.



Similar content being viewed by others
References
Patai S, Rappoport Z (eds) (1986/1987) The chemistry of organic selenium and tellurium compounds, vols. 1 and 2, Wiley
Maji S, Mukherjee N, Mondal A, Adhikary B, Karmakar B (2011) Inorg Chim Acta 371:21–26
Maji S, Mukherjee N, Dutaa AK, Srivastava DN, Paul P, Karmakar B, Mondal A, Adhikary B, Karmakar B (2011) Mater Chem Phys 130:392–397
Vittal J, Theivanayagam D (2002) Prog Crysl Growth Char Mat 45:21–27
Schenk WA, Sonnhalter N, Burzlaff N (1997) Z Naturforsch 52b:117–124
El-khateeb M, Jazzazi TMA, Görls H, Al-Shboul TMA, Westerhausen M (2011) Trans Met Chem 36:29–33
El-khateeb M, Asali KJ, Shaheen M, Rababa’ah A (2008) Jord J Chem 3:33–37
El-khateeb M, Görls H, Weigand W (2006) J Organomet Chem 691:5816–5820
El-khateeb M, Rüffer T, Lang T (2006) Polyhedron 25:3920–3924
El-khateeb M, Asali KJ, Abu Salem T, Welter R (2006) Inorg Chim Acta 359:4259–4264
Chaudhari K, Wadawle A, Jain VK, Vadav N, Bohar R (2010) Ind J Chem 49A:34–38
Chatuvedi SS, Bhattacharya J (2012) J Chem Soc Dalton Trans 41:424–431
Kedamath G, Kumbhare LB, Jain VK, Phadins PP, Nathaji M (2006) J Chem Soc Dalton Trans 2714–2718
Ng MD, Dean PA, Vittal JJ (2004) J Chem Soc Dalton Trans 2890–2894
El-Hinnawi MA, Ajlouni A (1987) J Organomet Chem 332:321–329
El-Hinnawi MA, Ajlouni A, Abu-Nasser JS, Powell AK, Wahrenkamp H (1989) J Organomet Chem 359:79–86
Jibril I, Abu Nimreh O (1996) Synth React Inorg Met-Org Chem 26:1409–1419
El-khateeb M, Görls H, Weigand W (2007) Inorg Chim Acta 306:705–709
Jibril I, Ali AK (1997) Ind J Chem 36A:987–991
Jibril I, Ali AK, Omar JT (1997) Polyhedron 16:3327–3331
El-Hinnawi MA, Aruffo AA, Santarsiero BD, McAlister DR, Shomaker V (1983) Inorg Chem 22:1585–1590
COLLECT (1998) Data collection software; Nonius BV, Netherlands
Processing of X-Ray Diffraction Data Collected in Oscillation Mode” Otwinowski Z, Minor W, in Carter CW, Sweet RM, (eds): (1997) Methods in Enzymology, Macromolecular Crystallography, Part A, 276:307–326, Academic Press
Sheldrick GM (2008) Acta Cryst A46:112–122
El-khateeb M, Obidate T (2001) Polyhedron 20:2393–2396
El-khateeb M (2004) Inorg Chim Acta 357:4341–4344
El-khateeb M (2006) Polyhedron 25:1386–1390
Jibril I, El-khateeb M, Barakat H, Rheinwald G, Lang H (2002) Inorg Chim Acta 333:1–6
El-khateeb M, Jibril I, Barakat H, Rheinwald G, Lang H (2003) Polyhedron 22:3445–3449
El-khateeb M, Shaver A, Lebuis A-M (2001) J Organomet Chem 622:293–296
Acknowledgments
The financial support (Grant no. 93/2011) from the Deanship of Scientific Research, Jordan University of Science and Technology is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
El-khateeb, M., Al-Noaimi, M., Al-Rejjal, N. et al. Mono- and bi-iron chalcogenocarboxylate complexes. Transition Met Chem 38, 529–534 (2013). https://doi.org/10.1007/s11243-013-9720-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11243-013-9720-x