Abstract
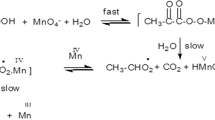
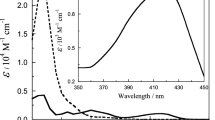
The kinetics of oxidation of malic acid by peroxomonosulphate (PMS) in the presence of Cu(II) (2.50 × 10−4–5.00 × 10−3 M), Co(II) (2.00 × 10−6–1.00 × 10−5 M) and Ni(II) (5.00 × 10−4–6.00 × 10−3 M) were studied in the pH range 4.05–5.89. The oxidation of Ni(II) malate follows simple first-order kinetics with respect to both [PMS] and [Ni(II)], while the oxidations of Cu(II) malate and Co(II) malate show autocatalysis. There is an appreciable induction period in the Cu(II) malate oxidation, while Co(II) malate oxidation follows a simple curve. The initial oxidation product for all three systems was identified as malonic semialdehyde. Alcohol quenching studies suggest that, even in the Co(II) malate-PMS system, no radical intermediates such as \( {\text{SO}}_{4}^{ - .} \) or \( {\text{OH}}{}^{.} \) are detected. The malonic semialdehyde intermediate may react with M(II) malates to give a hemiacetal, which may be more reactive.









Similar content being viewed by others
References
Sailani R, Sharma M, Pareek D, Khandelwal CL, Sharma PD (2012) Reac Kinet Mech Cat 105:249–259
Eor S, Hwang J, Choi MG, Chang S-K (2011) Org Lett 13:370–373
Chow TW-S, Wong EL-M, Guo Z, Liu Y, Huang J-S, Che C-M (2010) J Am Chem Soc 132:13229–13239
Lente G, Kalmar J, Baranyai Z, Kun A, Kek I, Bajusz D, Takacs M, Veres L, Fabian I (2009) Inorg Chem 48:1763–1773
Rivas FJ, Beltran FJ, Carvalho F, Alvarez PM (2005) Ind Eng Chem Res 44:749–758
Bennet JE, Gilbert BC, Stell JK (1991) J Chem Soc Perkin Trans 2:1105–1110
Anipsitakis GP, Dionysiou DD (2004) Environ Sci Technol 38:3705–3712
Spiro M (1979) Electrochim Acta 24:313–314
Furholz U, Haim A (1987) Inorg Chem 26:3243–3248
Eberson L (1982) Adv Phys Org Chem 18:79–185
Thendral P, Shailaja S, Ramachandran MS (2007) Int J Chem Kinet 39:320–327
Shailaja S, Ramachandran MS (2009) Int J Chem Kinet 41:160–167
Andal P, Murugavelu M, Shailaja S, Ramachandran MS (2009) Int J Chem Kinet 41:449–454
Shailaja S, Ramachandran MS (2011) Int J Chem Kinet 43:620–630
Miltenberger K (2005) Hydroxycarboxylic acids, aliphatic; Ullmann’s encyclopedia of industrial chemistry. Wiley-VCH, Weinheim
Mayes PA (1983) Harper’s review of biochemistry, 19th edn. In: Martin Jr DW, Mayes PA, Rodwell VW (eds) LANGE medical publications: California, Chapter 14
Maruthamuthu P, Neta P (1977) J Phys Chem 81:937–940
Feigl F (1956) Spot tests in organic analysis. Elsevier, New York, p 347
Shailaja S, Ramachandran MS (2011) Int J Chem Kinet 43:47
Robinson WG, Coon MJ (1963) In: Colowick SP, Kaplan NO (eds) Methods in enzymology, vol 6. Academic Press, New York, p 549
Kalnitsky G, Tapley DF (1958) Biochem J 70:28–34
Hayaishi O, Nishizuka Y, Tatibana M, Takeshita M, Kuno S (1961) J Biol Chem 236:781–790
Muller JG, Hickerson RP, Perez RJ, Burrows CJ (1977) J Am Chem Soc 119:1501–1506
Hickerson RP, Watkins-Sims CD, Burrows CJ, Atkins JF, Gesteland RF, Feldon B (1998) J Mol Biol 279:577–587
Stemmler AJ, Burrows CJ (2001) J Biol Inorg Chem 6:100–106
NIST critically selected stability constants of metal complexes: ver. 8.0
Smith RM, Martell AE (1974–1977, 1982, 1989) Critical stability constants, vols 1–6. Plenum, New York
Motekaitis RJ, Martell AE (1992) Inorg Chem 31:11–15
Campi E, Ostacoli G, Meirone M, Saini G (1964) J Inorg Nucl Chem 26:553–564
Rajan KS, Martell AE (1967) J Inorg Nucl Chem 29:463–471
Manning PG, Monk CB (1961) Trans Farad Soc 57:1996–1999
Ball DL, Edwards JO (1956) J Am Chem Soc 78:1125–1129
Goodman JF, Robson P (1963) J Chem Soc 2871–2875
Abrahamson HB, Rezvani AB, Brushmiller JG (1994) Inorg Chim Acta 226:117–127
Stone AT, Godtfredson KL, Deng DL (1994) In: Bidoglio G, Stumm W (eds) Chemistry of aquatic systems: local and global perspectives. Kluwer, Dordrecht, pp 337–374
Ferdousi BN, Islam MM, Awad MI, Okajima T, Kitamara F, Ohsaka T (2006) Electrochemistry 74:606–608
Ramachandran MS, Vivekanandam TS (1984) J Chem Soc Perkin Trans 2:1341–1344
Ramachandran MS, Vivekanandam TS, Raj RPMM (1984) J Chem Soc Perkin Trans 2:1345–1349
Lloyd D (1965) Biochem J 96:766–770
March J (1992) Advanced organic chemistry: reactions, mechanisms, and structure, 4th edn. Wiley-Interscience, Singapore, pp 889–891
Neta P, Huie RE, Ross AB (1988) J Phys Chem Ref Data 17:1027–1284
Buxton GV, Greenstock CL, Helman WP, Ross AB (1988) J Phys Chem Ref Data 17:513–886
Bratsch SG (1989) J Phys Chem Ref Data 18:1–21
Thompson RC, Wieland P, Appelman EH (1979) Inorg Chem 18:1974–1977
Acknowledgments
Murugavelu expresses his gratitude to UGC-RGNF, New Delhi for the financial assistance through a junior fellowship.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Murugavelu, M., Ramachandran, M.S. Kinetic studies of the oxidation of transition metal(II) malate complexes by peroxomonosulphate. Transition Met Chem 38, 225–234 (2013). https://doi.org/10.1007/s11243-012-9682-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11243-012-9682-4