Abstract
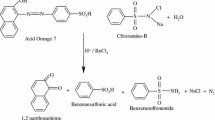
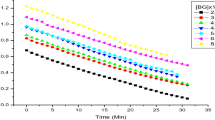
The kinetics and mechanism of sodium N-chloro-p-toluenesulfonamide oxidative decolorization of ethyl orange (EO) in aqueous perchloric acid have been studied at 303 K in the presence of rhodium(III) chloride as catalyst. The reaction exhibits first-order dependence on [EO]o and a fractional-order dependence on [CAT]o, [H+] and [RhIII]. The dielectric effect is positive. The stoichiometry of the reaction was found to be 1:1, and the oxidation products of EO were identified as N-(4-diethylamino-phenyl)-hydroxylamine and 4-nitroso-benzenesulfonic acid. The rhodium(III)-catalyzed reaction is about fourfold faster than the uncatalyzed reaction. The proposed mechanism and derived rate law are in agreement with the observed kinetic results.






Similar content being viewed by others
References
Jain AK, Gupta VK, Bhatnagar A, Suhas (2003) J Hazard Mater 101:31. doi:10.1016/S0304-3894(03)00146-8
Campbell MM, Johnson G (1978) Chem Rev 78:65. doi:10.1021/cr60311a005
Banerji KK, Jayaram B, Mahadevappa DS (1987) J Sci Ind Res 46:65
Kolaveri E, Ghorbeni-Choghamarani A (2007) J Iran Chem Soc 2:126
Agnihotri G (2005) Synlett 18:2857. doi:10.1055/s-2055-918936
Puttaswamy, Anuradha TM, Ramachandrappa R, Gowda NMM (2000) Int J Chem Kinet 32:221
Ramachandra H, Rangappa KS, Mahadevappa DS, Gowda NMM (1996) Monatsh Chem 127:241
Puttaswamy, Sukhdev A, Shubha JP (2009) J Mol Catal A: Chem 310:24. doi:10.1016/j.molca.2009.05.015
Jagadeesh RV, Puttaswamy (2008) J Phys Org Chem 21:844. doi:10.1002/poc.1379
Vinod KN, Puttaswamy, Gowda KNN (2010) Ind Eng Chem Res 49:3137. doi:10.1021/1e90062801
Morris JC, Salazar JA, Wineman MA (1948) J Am Chem Soc 70:2036. doi:10.1021/ja01186a016
Akreloff G (1932) J Am Chem Soc 54:4125
Bishop E, Jennings VJ (1958) Talanta 1:197. doi:10.1016/0039-9140(58)80034-X
Hardy FF, Johnston JP (1973) J Chem Soc Perkin Trans II(2):742
Wolsey WC, Reynolds CA, Kleinberg J (1963) J Inorg Chem 2:463
Radakrishnamurthy PS, Misra SA (1982) Int J Chem Kinet 14:631
James BR, Rempel GL (1966) Can J Chem 44:233
Singh AK, Singh RK, Srivastava J, Rahamani S, Yadav S (2012) Indian J Chem 51A:681
Praveen KT, Gayatri, Sumitha S, Manish S, Santhosh BS (2007) Appl Organomet Chem 21:135. doi:10.1002/aoc.1169
Singh B, Singh AK, Singh A (2011) Indian J Chem 50A:650
Amis ES, Jaffe G (1942) J Chem Phys 10:598. doi:10.1063/1.1723770
Kirkwood JG (1934) J Chem Phys 2:351
Laidler KJ, Landskroener PA (1957) Trans Faraday Soc 52:200
House JE (1997) Principles of chemical kinetics. WMC-Brown Publishers, Boston
Reichardt C (2003) Solvent and solvent effects in organic chemistry, 3rd edn. Wiley, NewYork
Laidler KJ (2006) Chemical kinetics, 3rd edn. Harper and Row Publishers Inc, New York, pp 79–114
Moelwyn-Hughes EA (1947) Kinetics of reactions in solutions. Oxford University Press, London, pp 297–299
Acknowledgments
The authors greatly acknowledge the University Grant Commission, New Delhi for the award of UGC-Major Research Project. We thank Prof. M. A. Pasha of this department for his valuable suggestion on the reaction scheme.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Manjunatha, A.S., Puttaswamy Rhodium(III) as a homogeneous catalyst for the oxidative decolorization of ethyl orange with aqueous acidic chloramine-T: a spectrophotometric, kinetic and mechanistic study. Transition Met Chem 38, 183–190 (2013). https://doi.org/10.1007/s11243-012-9676-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11243-012-9676-2