Abstract
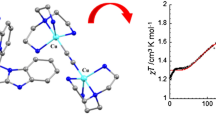
Four pyridinecarboxamide iron dicyanide building blocks and one Mn(III) compound have been employed to assemble cyanide-bridged heterometallic complexes, resulting in a series of trinuclear cyanide-bridged FeIII–MnII complexes: {[Mn(DMF)2 (MeOH)2][Fe(bpb)(CN)2]2}·2DMF (1), {[Mn(MeOH)4][Fe(bpmb)(CN)2]2}·2MeOH·2H2O (2), {[Mn(MeOH)4][Fe(bpdmb)(CN)2]2}·2MeOH·2H2O (3) and {[Mn(MeOH)4][Fe(bpClb)(CN)2]2}·4MeOH (4) (bpb2− = 1,2-bis(pyridine-2-carboxamido)benzenate, bpmb2− = 1,2-bis(pyridine-2-carboxamido)-4-methyl-benzenate, bpdmb2− = 1,2-bis(pyridine-2-carboxamido)-4,5-dimethyl-benzenate, bpClb2− = 1,2-bis(pyridine-2-carboxamido)-4-chloro-benzenate). Single-crystal X-ray diffraction analysis shows their similar sandwich-like structures, in which the two cyanide-containing building blocks act as monodentate ligands through one of their two cyanide groups to coordinate the Mn(II) center. Investigation of the magnetic properties of these complexes reveals antiferromagnetic coupling between the neighboring Fe(III) and Mn(II) centers through the bridging cyanide group. A best fit to the magnetic susceptibilities of complexes 1 and 3 gave the magnetic coupling constants J = −1.59(2) and −1.32(4) cm−1, respectively.




Similar content being viewed by others
References
Kahn O (1993) Molecular magnetism. VCH, Weinheim
Sato O, Kawakami T, Kimura M, Hishiya S, Kubo S, Einaga Y (2004) J Am Chem Soc 126:13176
Shatruk M, Dragulescu-Andrasi A, Chambers KE, Stoian SA, Bominaar EL, Achim C, Dunbar KR (2007) J Am Chem Soc 129:6104
Lu Z, Wang X, Liu Z, Liao B, Gao S, Xiong R, Ma H, Zhang D, Zhu D (2006) Inorg Chem 45:999
Kurmoo M, Kumagai HM, Hughes SJ, Kepert C (2003) Inorg Chem 42:6709
Galet A, Muñoz MC, Real JA (2006) Inorg Chem 45:4583
Agustí G, Muñoz MC, Real JA (2008) Inorg Chem 47:2552
Agustí G, Muñoz MC, Gaspar AB, Real JA (2009) Inorg Chem 48:3371
Long J, Chamoreau LM, Mathoniére C, Marvaud V (2009) Inorg Chem 48:22
Bleuzen A, Marvaud V, Mathoniere C, Sieklucka B, Verdaguer M (2009) Inorg Chem 48:3453, and references therein
Atanasov M, Comba P, Daul CA (2008) Inorg Chem 47:2449
Atanasov M, Busche C, Comba P, Hallak FE, Martin B, Rajaraman G, van Slageren J, Wadepohl H (2008) Inorg Chem 47:8112
Kim J II, Kwak HY, Yoon JH, Ryu DW, Yoo IY, Yang N, Cho BK, Park JG, Lee H, Hong CS (2009) Inorg Chem 48:2956
Yeung WF, Lau PH, Wang XY, Gao S, Szeto L, Wong WT (2006) Inorg Chem 45:6756
Bleuzen A, Marvaud V, Mathoniere C, Sieklucka B, Verdaguer M (2009) Inorg Chem 48:3453–3466
Herrera JM, Marvaud V, Verdaguer M, Marrot J, Kalisz M, Mathoniére C (2004) Angew Chem Int Ed 43:5468
Ni ZH, Zhang LF, Tangoulis V, Wernsdorfer W, Cui AL, Sato O, Kou HZ (2007) Inorg Chem 46:6029
Ni ZH, Kou HZ, Zhang LF, Ge C, Cui AL, Wang RJ, Li Y, Sato O (2005) Angew Chem Int Ed 44:7742
Kou HZ, Ni ZH, Liu CM, Zhang DQ, Cui AL (2009) New J Chem 33:2296
Ni ZH, Kou HZ, Zhao YH, Zheng L, Wang RJ, Cui AL, Sato O (2005) Inorg Chem 44:2050
Ray M, Mukherjee R, Richardson JF, Buchanan RM (1993) J Chem Soc Dalton Trans 2451
Stults RB, Marianelli RS, Day VW (1975) Inorg Chem 14:722
Zhang DP, Wang HL, Chen YT, Ni ZH, Tian LJ, Jiang JZ (2009) Inorg Chem 48:5488
Zhang DP, Wang HL, Chen YT, Ni ZH, Tian LJ, Jiang JZ (2009) Dalton Trans 9418
Ni ZH, Tao, J, Wernsdorfer W, Cui AL, Kou HZ (2009) J Chem Soc Dalton Trans 2788
Acknowledgments
This work was supported by the Fundamental Research Funds for the Central Universities and the Doctoral Starting Fund of Shandong University of Technology (410027).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhang, D., Zhang, L., Chen, X. et al. A series of trinuclear sandwich-like cyanide-bridged iron(III)-manganese(II) complexes: synthesis, crystal structures, and magnetic properties. Transition Met Chem 36, 539–544 (2011). https://doi.org/10.1007/s11243-011-9500-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11243-011-9500-4