Abstract
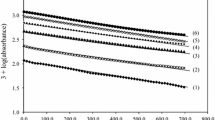
The oxidation of d-panthenol by MnO4 − was studied in the absence and in the presence of ruthenium(III) catalyst in alkaline medium at 298 K and at constant ionic strength of 0.50 mol dm−3 by spectrophotometry. The stoichiometry in both the cases was [panthenol]: [MnO4 −] = 1:4. The oxidation products were identified by IR and GC–MS. The reaction was first-order with respect to both MnO4 − and ruthenium(III), while the orders with respect to both panthenol and alkali varied from first order to zero order as the concentrations increased. The effects of added products, ionic strength and dielectric constant were studied. The reaction constants, activation parameters and thermodynamic quantities were calculated for both the uncatalysed and catalysed reactions.










Similar content being viewed by others
References
Caron P, Dugger RW, Ruggeri SG, Ragan JA, Brown Ripin DH (2006) Pharm Ind Chem Rev 106:2943
Lee DG (1980) The oxidation of organic compounds by permanganate ion and hexavalent chromium, open court. La Salle IL, lllinois
Perez-Benito JF, Lee DG (1987) J Org Chem 52:3239
Simandi LI, Jasky M, Savage CR, Schelly ZA (1985) J Am Chem Soc 107:4220
Das AK (2001) Coord Chem Revs 213:307
Savanur AP, Nandibewoor ST, Chimatadar SA (2009) Transition Met Chem 34:711
Abiko Y (1969) J Vitamin 15:59
Lewis J, Gonzales GD, Winck CL (1977) Therapeutic-index pharmocopie, official monographs for part I, JP XIV. The Pharmaceutical Press, London, p 588
Jeffery GH, Bassett J, Mendham J, Denny RC (1996) Vogel’s text book of quantitative chemical analysis, 5th edn. ELBS Longman, Essex, p 370
Carrington A, Symons MCR (1956) J Chem Soc 337:3376
Kamble DL, Chougale RB, Nandibewoor ST (1996) Indian J Chem 35A:865
Feigl F, Anger V (1975) Spot tests in organic analysis. Elsevier, Oxford, p 469
Moelwyn-Hughes EA (1947) Kinetics of reaction in solutions. Oxford University Press, London, p 297
Thabaj KA, Kulkarni SD, Chimatadar SA, Nandibwoor ST (2007) Polyhedron 26:4877
Chimatadar SA, Kini AK, Nandibwoor ST (2003) Indian J Chem A42:1850
Laidler KJ (2004) Chemical kinetics, 3rd edn. Pearson Education ptc. Ltd, New Delhi, p 183
Amis ES (1966) Solvent effects on reaction rates and mechanisms. New York, Academic Press
Bugarcic ZD, Nandibewoor ST, Hamza MSA, Heimemann F, Eldik RV (2006) Dalton Trans 29:84
Singh HS, Singh RK, Singh SM, Sisodia AK (1977) J Phy Chem 81:1044
Kiran TS, Hiremath CV, Nandibewoor ST (2006) Appl cat A 305:79
Rao SV, Jagannadham V (1985) Ract kinet Catal Lett 27:239
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hosahalli, R.V., Savanur, A.P., Nandibewoor, S.T. et al. Kinetics and mechanism of uncatalysed and ruthenium(III)-catalysed oxidation of d-panthenol by alkaline permanganate. Transition Met Chem 35, 237–246 (2010). https://doi.org/10.1007/s11243-009-9319-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11243-009-9319-4