Abstract
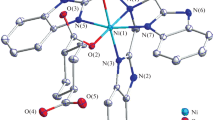
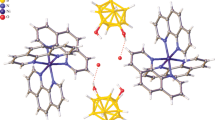
The products obtained by the reactions of Ni(OAc)2·4H2O with Hpot (Hpot = 5-phenyl-1,3,4-oxadiazole-2-thione) and [K(H2fchc)] (potassium N′-(furan-2-carbonyl) hydrazine carbodithioate), on treatment with excess of ethylenediamine (en), gave mixed ligand complexes [Ni(pot)2(en)2] (1) and [Ni(fot)2(en)2] (2) (fot = 5-furan-(1,3,4)-oxadiazole-2-thione). These complexes have been characterized with the aid of elemental analyses, IR, magnetic susceptibility and single crystal X-ray studies. In both complexes, the heterocyclic ligand coordinates through oxadiazole nitrogen, and the ligand exists as the thione form. The complexes 1 and 2 have distorted octahedral geometries around the centrosymmetric Ni(II) center with trans oxadiazole ligands. Both complexes show extended hydrogen bonding to give a supramolecular framework.






Similar content being viewed by others
References
Hassert GL, Poutsiava JW, Papadrianos D, Barke JC, Graver BN (1961) Taxicol Appl Pharmocol 3:726. doi:10.1016/0041-008X(61)90036-9
Mohsen A, Omer ME, Aboulwafa OM (1984) J Heterocycl Chem 21:1415. doi:10.1002/J.het.5570210538
Jakubkiene V, Burbuliene MM, Mekuskiene G, Udrenaite E, Gaidelis P, Vainilavicius P (2003) Il Farmaco 58:323. doi:10.1016/S0014-827X(02)00022-8
Dutta MM, Goswami BN, Kataky JCS (1986) J Hetro Chem 23:793. doi:10.1002/J.Hetrocyclic5570230328
Mir I. Siddiqui MT, Comrie AM (1971) J Chem Soc C:2798. doi: 10.1039/J39710002798
Amin OH, Al-Hayaly LJ, Al-Jibori SA, Al-Allaf TAK (2004) Polyhedron 23:2013. doi:10.1016/J.poly.2004.05.006
Tripathi P, Pal A, Jancik V, Pandey AK, Singh J, Singh NK (2007) Polyhedron 26:2597. doi:10.1016/J.poly.2006.12.046
Xu HX, Ma JP, Huang RQ, Dong YB (2005) Acta Cryst E61:m2462. doi:10.1107/S1600536805034987
Wang YT, Tang GM (2007) Inorg Chem Commun 10:53. doi:10.1016/Jinoche.2006.09.010
Du M, Zhang ZH, Zhao XJ, Xu Q (2006) Inorg Chem 45:5785. doi:10.1021/ic060129v
Wang YT, Tang GM, Ma WY, Wan WZ (2007) Polyhedron 26:782. doi:10.1016/J.poly.2006.09.008
Zhang ZH, Li CP, Tang GM, Tian YL, Guo YM (2008) Inorg Chem Commun 11:326. doi:10.1016/J.poly.2006.09.008
Zhang ZH, Tian YL, Guo YM (2007) Inorg Chim Acta 360:2783. doi:10.1016/Jica.2006.11.020
Singh M, Butcher RJ, Singh NK (2008) Polyhedron 27:3151. doi:10.1016/Jpoly.2008.06.038
Wang YT, Tang GM, Qiang ZW (2007) Polyhedron 26:4542. doi:10.1016/Jpoly.2007.06.026
Vogel AI (1969) A text book of quantitative inorganic analysis, 3rd edn. ELBS, Longman, London
Lee RH, Griswold G, Kleinberg H (1964) Inorg Chem 3:1278. doi:10.1021/ic50019a019
Singh NK, Butcher RJ, Tripathi P, Srivastava AK, Bharty MK (2007) Acta Cryst E63:o782. doi:10.1107/S1600536806052238
Sheldrick GM (2008) Acta Cryst A64:112. doi:10.1107/S0108767307043930
Bruno IJ, Cole JC, Edgington PR, Kessler M, Macrae CF, McCabe P, Pearson J, Taylor R (2002) Acta Crystallogr Sect B58:389. doi:10.1107/S0108768102003324
Brandenburg K, Putz H (2004) Diamond version 3.0. University of Bonn, Germany
Farrugia LJJ (1997) Appl Crystallogr 30:565. doi:10.1107/S0021889897003117
Molina P, Tarraga A, Espinosa A (1988) Synthesis 9:690. doi:10.1055/s-1988-27672
Patricia GS, Javier GT, Miguel AM, Francisco JA, Teofilo R (2002) Inorg Chem 41:1345. doi:10.1021/ic015625s
Lever ABP (1984) Inorganic electronic spectroscopy, 2nd edn. Elsevier, Amsterdam
Terron A, Rosa AG, Fiol JJ, Amengual S, Oliver MB, Totaro RM, Apella MC, Molins E, Mata I (2004) J Inorg Biochem 98:632. doi:10.1016/J.Jinorgbio.2004.02.002
Squattrito PJ, Iwamoto T, Nishikiori SI (1996) Chem Commun 23:2665. doi:10.1039/cc9960002665
Icbudak H, Olmez H, Yesilel OZ, Arslan F, Naumov P, Jovanovski G, Ibrahim AR, Usman A, Fun HK, Chantrapromma S, Ng SW (2003) J Mol Struct 657:255. doi:10.1016/S0022-2860(03)00404-6
Acknowledgments
Authors thank CSIR, New Delhi for financial support by grant No. 01 (2152)07/EMR-II.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Singh, N.K., Bharty, M.K., Kushawaha, S.K. et al. Nickel(II) complexes of 5-phenyl and 5-furan-1,3,4-oxadiazole-2-thiones containing ethylenediamine: synthesis, spectral and X-ray characterization. Transition Met Chem 35, 205–211 (2010). https://doi.org/10.1007/s11243-009-9315-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11243-009-9315-8