Abstract
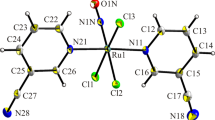
The solvento species obtained by the treatment of cis-RuCl2(N,N-L)2 [L = di-2-pyridyl sulfide (dps), di-2-pyrimidyl sulfide (dprs)] with AgPF6, reacted with dithioethers L′ [L′ = 2,6-bis(2-pyridylthiomethyl)pyridine (pytmp), 2,6-bis(2-pyrimidylthiomethyl)pyridine (prtmp) and 2,6-bis{2-(4-methyl)pyrimidylthiomethyl} pyridine (mprtmp)] to afford the compounds [Ru(N,N-L)2(N,S-L′)][PF6]2. The 1H NMR spectra indicate that L′ is chelated through S and N atoms with the formation of a four-membered ring. As a consequence, the ruthenium and sulfur atoms are stereogenic centers with ∆ and Λ and (R) and (S) configurations, respectively. NMR spectra, at low temperatures, show that two invertomers, of similar abundance, as enantiomeric couples ∆S, ΛR and ∆R, ΛS are present. In the methylene region, four AB systems are observed that in both the species contain two non-equivalent methylene groups. Variable-temperature NMR spectra and EXSY experiments show that the sulfur inversion produces an exchange between the invertomers. The one-dimensional band-shape analysis of the exchanging methylene signals showed that the energy barriers for the process are in the 43–52 kJ mol−1 range. The possible mechanisms of the sulfur inversion are discussed.




Similar content being viewed by others
References
Bai S-Q, Koh LL, Hor TSA (2009) Inorg Chem 48:1207–1213
Gennari M, Tegoni M, Lanfranchi M, Pellinghelli MA, Giannetto M, Marchiò L (2008) Inorg Chem 47:2223–2232
Heard PJ (2007) Chem Soc Rev 36:551–569
Huynh HV, Yeo CH, Tan GK (2006) J Chem Soc Chem Commun 3833–3835
Braunstein P, Naud F (2001) Angew Chem Int Ed Engl 40:680–699
Slone CS, Weimberg DA, Mirkin CA (1999) Prog Inorg Chem 48:233–350
Scopelliti R, Bruno G, Donato C, Tresoldi G (2001) Inorg Chim Acta 313:43–55
Tresoldi G, Lo Schiavo S, Lanza S, Cardiano P (2002) Eur J Inorg Chem 181–191
Baradello L, Lo Schiavo S, Nicolò F, Lanza S, Alibrandi G, Tresoldi G (2004) Eur J Inorg Chem 3358–3369
Tresoldi G, Baradello L, Lanza S, Cardiano P (2005) Eur J Inorg Chem 2423–2435
Tresoldi G, Lanza S, Di Pietro S, Drommi D (2008) Eur J Inorg Chem 4807–4815
Bruno G, Nicolò F, Lo Schiavo S, Sinicropi MS, Tresoldi G (1995) J Chem Soc Dalton Trans 17–24
Tresoldi G, Piraino P, Rotondo E, Faraone F (1991) J Chem Soc Dalton Trans 425–430
Tresoldi G, Lo Schiavo S, Piraino P, Zanello P (1996) J Chem Soc Dalton Trans 885–892
Bruno G, Rotondo A, Tresoldi G, Nicolò F, Lanza S (2005) Acta Crystallogr C 61:M169–M171
Abel EW, Ellis D, Orrel KG (1992) J Chem Soc Dalton Trans 2243–2249
Abel EW, Heard PG, Orrel KG, Hursthouse B, Mazid MA (1993) J Chem Soc Dalton Trans 3795–3801
Abel EW, Blackwall ES, Creber ML, Heard PG, Orrel KG (1995) J Organomet Chem 490:83–88
Abel EW, Long NJ, Orrel KG, Osborne AG, Pain HM, Sik V (1992) J Chem Soc Chem Commun 303–304
Abel EW, Dimitrov VS, Long NJ, Orrel KG, Osborne AG, Pain HM, Sik V, Hursthouse MB, Mazid MA (1993) J Chem Soc Dalton Trans 291–298
Abel EW, Dimitrov VS, Long NJ, Orrel KG, Osborne AG, Pain HM, Sik V, Hursthouse MB, Mazid MA (1993) J Chem Soc Dalton Trans 597–603
Abel EW, Orrel KG, Osborne AG, Pain HM, Sik V (1994) J Chem Soc Dalton Trans 111–116
Abel EW, Hylands KA, Olsen MD, Orrel KG, Osborne AG, Sik V, Ward GN (1994) J Chem Soc Dalton Trans 1079–1090
Gelling A, Noble DR, Orrel KG, Osborne AG, Sik V (1996) J Chem Soc Dalton Trans 3065–3070
Gelling A, Olsen MD, Orrel KG, Osborne AG, SiK V (1998) J Chem Soc Dalton Trans 3479–3488
Heard PJ, Cameron J (1997) J Chem Soc Dalton Trans 1083–1091
Heard PJ, King PM, Bain AD, Hazendonk P, Tocher DA, Cameron J (1999) J Chem Soc Dalton Trans 4495–4501
Rotondo E, Giordano G, Minniti D (1996) J Chem Soc Dalton Trans 253–254
Groen JH, van Leeuwen PWNM, Brieze K (1998) J Chem Soc Dalton Trans 113–117
Binsch G, Kesseler H (1980) Angew Chem Int Ed Engl 19:411–428
Chachaty C, Pappalardo C, Scarlata CG (1976) J Chem Soc Perkin Trans 2:1234–1238
Joseph-McCarthy D, Tsang SK, Filman DJ, Hogle MJ, Karplus M (2001) J Am Chem Soc 123:12758–12769
Peng R, Li D, Wu T, Zhou XP, Ng SW (2006) Inorg Chem 45:4035–4046
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
11243_2009_9308_MOESM2_ESM.tif
Variable-temperature 1H NMR spectra of [Ru(dps)2(mprtmp)][PF6]2 (4); computer synthesized spectra are shown on the right (TIFF 1433 kb)
Rights and permissions
About this article
Cite this article
Tresoldi, G., Di Pietro, S., Drommi, D. et al. Fluxional rearrangement in congested ruthenium(II) complexes containing pyrimidine- and/or pyridine-based dithioether ligands. Transition Met Chem 35, 151–158 (2010). https://doi.org/10.1007/s11243-009-9308-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11243-009-9308-7