Abstract
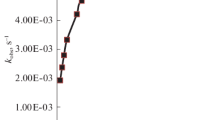
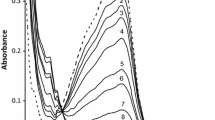
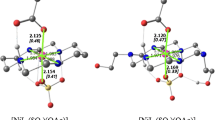
The formation of CuII–Br–FeIII-type heterobimetallic complexes was observed spectrophotometrically, given the non-additivity of the spectra from the copper(II) and iron(III) complexes. The kinetics of the oxidation of promazine radical (ptz+•) to promazine 5-oxide, by iron(III) bromides, copper(II) bromides, and a mixture of these complexes in acidic aqueous solutions, have been studied using UV–Vis spectroscopy at I = 1.0 M (H+, Cu2+, Fe3+, Br−) and T = 318 K. Copper(II) inhibits the oxidation of the promazine radical to promazine sulfoxide using iron(III) complexes. A rate retardation effect, characterized by the dependence of the pseudo second-order rate constant (k II) on the copper(II) concentration k II = a/(1 + b[CuII]), can be rationalized as a result of CuII–Br–FeIII-type heterobimetallic complex formation.






Similar content being viewed by others
References
Wang S, Ferbinteanu M, Yamashita M et al (2007) Inorg Chem 46:610. doi:10.1021/ic061681n
Gillon B, Goujon A, Willemin S, Larionova J, Desplanches C, Ruiz E, André G, Stride JA, Guérin CH et al (2007) Inorg Chem 46:1090. doi:10.1021/ic0611645
Shatruk M, Dragulescu-Andrasi A, Chambers KE, Stoian SA, Bominaar EL, Achim C, Dunbar KR et al (2007) J Am Chem Soc 129:6104. doi:10.1021/ja066273x
Ward MD et al (2007) Coord Chem Rev 251:1663. doi:10.1016/j.ccr.2006.10.005
Fernández EJ, Laguna A, López-de-Luzuriaga JM et al (2007) Dalton Trans 1969. doi:10.1039/b702838p
Kabayashi M, Takashima A, Ishii T, Naka H, Uchiyama M, Yamaguchi K et al (2007) Inorg Chem 46:1039. doi:10.1021/ic0616986
Esswein AJ, Dempsey JL, Nocera DG et al (2007) Inorg Chem 46:2362. doi:10.1021/ic062203f
Neves A, Lanznaster M, Bortoluzzi AJ, Peralta RA, Casellato A, Castellano EE, Herrald P, Riley MJ, Schenk G et al (2007) J Am Chem Soc 129:7486. doi:10.1021/ja071184l
Cox RS, Schenk G, Mitic N, Gahan LR, Hengge AC et al (2007) J Am Chem Soc 129:9550. doi:10.1021/ja072647q
Chufán EE, Mondal B, Gandhi T, Kim E, Rubie ND, Moënne-Loccoz P, Karlin KD et al (2007) Inorg Chem 46:6382. doi:10.1021/ic700363k
Ritleng V, Chetcuti MJ et al (2007) Chem Rev 107:797. doi:10.1021/cr940270y
Seymore SB, Brown SN et al (2006) Inorg Chem 45:9540. doi:10.1021/ic061153b
Ma R, Liu Z, Takada K, Iyi M, Bando Y, Sasaki T et al (2007) J Am Chem Soc 129:5257. doi:10.1021/ja0693035
Topolski A, Marai H, Chatłas J, Kita P et al (2006) Polish J Chem 80:503
Topolski A, Kita P, Katafias A et al (2007) Trans Met Chem 32:1126. doi:10.1007/s11243-007-0291-6
Richens DT et al (2005) Chem Rev 105:1961. doi:10.1021/cr030705u
Wiśniewska J, van Eldik R et al (2002) Inorg Chem 41:3802. doi:10.1021/ic0201349
Wiśniewska J, Kita P, Wrzeszcz G et al (2007) Trans Met Chem 32:857. doi:10.1007/s11243-007-0219-1
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Topolski, A., Lipińska, M., Kita, P. et al. Kinetic studies on promazine oxidation by FeIII/CuII in acidic aqueous bromide solutions. Spectroscopic and kinetic non-additivity as evidence for the CuII–Br–FeIII-type heterobimetallic complex formation. Transition Met Chem 33, 843–847 (2008). https://doi.org/10.1007/s11243-008-9120-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11243-008-9120-9