Abstract
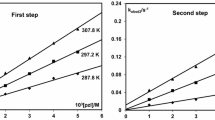
The kinetics of the reaction between [ReN(H2O)-(CN)4]2− with different κ2 N,O-donor ligands (quin− and 2,3-dipic−, respectively) have been studied in the pH 4–12 range in aqueous solution. Two consecutive reaction steps with the formation of the [ReN(η1-quin)(CN)4]3− and [ReN(μ2-quin) (CN)3]2− complexes, respectively, were spectrophotometrically observed and kinetically investigated. The same reaction mechanism is proposed for these two ligands. The first fast reaction (for quin−) is attributed to the aqua substitution of [ReN(H2O)(CN)4]2− with forward and reverse rate constants of 1.96(5) × 10−1 M−1 s−1 and 5.6(3) × 10−2 s−1, while a rate of 2.64(3) M−1 s−1 was observed for the reaction between the conjugate base [ReN(OH)(CN)4]3− and quin− at 40.2 °C. Due to small absorbance changes, it was difficult to obtain any good quality data for the fast reactions for 2,3-dipic−. The second, slower reaction is attributed to cyano substitution with rate constants (k 3 K 1) of 4.17(4) × 10−3 for quin− and 4.68(7) × 10−3 M−1 s−1 for 2,3-dipic−, at 80.02 °C, respectively. The acid dissociation constant for the aqua complex was spectrophotometrically determined as 11.58(3) and 11.54(2) and kinetically as 11.51(8) and 11.41(1), at 80.4 °C, respectively. Negative \(\Updelta{S}^{\neq}\) values of −83.5(2) and −144.1(2) J K−1 mol−1 as well as the \(\Updelta {H}^{\neq}\) of 71.4(3) and 47.3(3) kJ mol−1, for the slow quin− and 2,3-dipic− reactions, respectively, point to an ordered transition state where bond formation is responsible for the major driving force of the reaction. The \(\Updelta{H}^{\neq}=39.6(2)\,\hbox{kJ}\,\hbox{mol}^{-1}\) and \(\Updelta{S}^{\neq}=-142.8(1)\,\hbox{J\,K}^{-1}\,\hbox{mol}^{-1}\) for the fast forward reaction of quin− is indicative of expected associative activation in the transition state.










Similar content being viewed by others
References
Leipoldt JG, Basson SS, Roodt A, Purcell W (1992) Polyhedron 11:2277
Samotus A, Szklarzewicz J (1993) Coord Chem Rev 125:63
Leipoldt JG, Basson SS, Roodt A (1993) In: Sykes AG (ed) Advances in inorg. chem, vol 40, p 241
Kanas A, Dudek M, Samotus A (1976) Bull Acad Polon Sci Ser Sci Chim 24:43
Roodt A, Leipoldt JG, Basson SS, Potgieter IM (1988) Transition Met Chem 13:336
Roodt A, Leipoldt JG, Deutsch EA, Sullivan JC (1992) Inorg Chem 31:1080
Purcell W, Roodt A, Basson SS, Leipoldt JG (1991) Transition Met Chem 16:60
Damoense LJ, Purcell W, Leipoldt JG (1994) Transition Met Chem 19:619
Wieghardt K, Backes-Dahman G, Holzbach WJ, Swiridoff WJ, Weiss J (1983) Z Anorg Allg Chem 499 :44
Basson SS, Leipoldt JG, Potgieter IM, Roodt A (1985) Inorg Chim Acta 103:121
Leipoldt JG, Basson SS, Roodt A, Potgieter IM (1986) S Afr J Chem 39:179
Roodt A, Leipoldt JG, Basson SS, Potgieter IM (1990) Transition Met Chem 15:439
Purcell W, Damoense LJ, Leipoldt JG (1992) Inorg Chim Acta 195:217
Purcell W, Potgieter IM, Damoense LJ, Leipoldt JG (1991) Transition Met Chem 16:473
Basson SS, Leipoldt JG, Potgieter IM (1984) Inorg Chim Acta 90:57
Samotus A, Szklarzewicz J, Alcock NW (1990) Inorg Chim Acta 172:129
Leipoldt JG, Basson SS, Roodt A, Potgieter IM (1986) Transition Met Chem 11:323
Roodt A, Basson SS, Leipoldt JG (1994) Polyhedron 13:599
Leipoldt JG, Basson SS, Potgieter IM, Roodt A (1987) Inorg Chem 26:57
Samotus A, Kanas A, Glug W, Szklarzewicz J (1991) Transition Met Chem 16:614
Mtshali TN, Purcell W, Visser HG, Basson SS (2006) Polyhedron 25:2415
Okobu N, Muranishi Y (2003) Acta Cryst C59:m228
Zhang H-T, Chen Y-Q, Xu L, You X-Z (2003) Acta Cryst C59:m373
Zhang H-T, You X-Z (2003) Acta Cryst C59:m313
Xiang J-F, Li M, Wu S-M, Yuan L-J, Sun J-T (2006) Acta Cryst C62:m122
Mtshali TN, Purcell W, Visser HG, Basson SS (2007) Acta Cryst E63:m1037
Johnson NP (1969) J Chem Soc A 1843
Purcell W, Potgieter IM, Damoense LJ, Leipoldt JG (1992) Transition Met Chem 17:387
Mtshali TN, Purcell W, Visser HG, Basson SS (to be published)
Martell EA, Smith RM (1982) Critical stability constants. Plenum Press, p 129
Harmon KM, Brown PW, Gill SH (1988) J Mol Struct 448:43
Margerum DW, Caley GR, Weatherburn DC, Pakenkopf GW (1978) Coord Chem 2, Am Chem Soc 42
Purcell W, Roodt A, Basson SS, Leipoldt JG (1989) Transition Met Chem 14:224
Purcell W, Roodt A, Leipoldt JG (1991) Transition Met Chem 16:339
Purcell W, Roodt A, Basson SS, Leipoldt JG (1989) Transition Met Chem 14:5
Purcell W, Roodt A, Basson SS, Leipoldt JG (1990) Transition Met Chem 15:239
Manoli J-M, Potvin C, Bregeault J-M (1980) Dalton Trans 192
Acknowledgment
The authors thank the Andrew Mellon Foundation (T.N. Mtshali) and Research Fund of the University of the Free State for financial support.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Mtshali, T.N., Purcell, W., Visser, H.G. et al. Kinetic study of reaction of [ReN(H2O)(CN)4]2− with quinoline-2-carboxylate and pyridine-2,3-dicarboxylate anions. Transition Met Chem 33, 481–491 (2008). https://doi.org/10.1007/s11243-008-9068-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11243-008-9068-9