Abstract
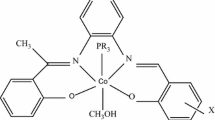
A u.v.–vis spectrophotometric study of the adduct formation of the nickel(II) Schiff base complexes,([NiL]) where L = [3-methoxysalophen, N,N′-bis(3-methoxysalicylidene)-1,2-phenylenediimine] (1), [4-methoxysalophen, N,N′-bis(4-methoxysalicylidene)-1,2-phenylenediimine] (2), [5-methoxysalophen, N,N′-bis(5-methoxysalicylidene)-1,2-phenylenediimine] (3) and [Salophen, [N,N′-bis(salicylaldehydo)-1,2-phenylenediimine] (4) as donors with R2SnCl2 (R = methyl, phenyl and n-butyl) as acceptors have been investigated in chloroform, as solvent. Adducts have been characterized by 1H, 13C and 119Sn NMR, IR and electronic spectroscopy and CHN elemental microanalysis. The formation constants and the thermodynamic free energies were measured using u.v.–vis spectrophotometry titration for 1:1 adduct formation at various temperatures (T = 278 to 308 K). The trend of the adduct formation of the nickel Schiff base complexes with a given tin acceptor decreases as follows:
and
The trend of the reaction of R2SnCl2 acceptors toward a given nickel Schiff base complex is as follows:
Similar content being viewed by others
References
Cimernman Z., Galic N. and Bosner B. (1997). Anal. Chim. Acta 343: 145
Raptopoulou C.P., Papadopoulos A.N., Malamatari D.A., Loannidis E., Molsidis G., Terzis A. and Kessissoglou D.P. (1998). Inorg. Chim. Acta 272: 283
Pignatello R., Panicol A., Mazzone P., Pinizzotto M., Garozzo A. and Furneri P. (1994). Eur. J. Med. Chem. 29: 781
Guofa L., Tongshun S. and Yonghian Z. (1997). J. Mol. Struct. 412: 75
Calligaris M. and Randaccio L. (1987). Comprehensive Coordination Chemistry. Pergamon press, London, vol. 2, Chap. 20
D. Cunningham, J. Fitzgerald and M. Little, J. Chem. Soc. Dalton Trans., 2261 (1987).
D. Cunningham, J.F. Gallagher, T. Higgins, P. McArdle, McGinley J. and M. O’Gara, J. Chem Soc. Dalton Trans., 2183 (1993).
D. Cunningham, B. Clarke, J.F. Gallagher, T. Higgins, P. McArdle, J. McGinley, M. Ni Cholchuin and D. Sheerin, J. Chem Soc. Dalton Trans., 2473 (1994).
Cunningham D., Boyce M., Clarke B., Gallagher J.F., Higgins T., McArdle P., McGinley J., Ni Cholchuin M. and O’Gara M. (1995). J. Organometallic Chem. 498: 241
Cunningham D., Clarke B., Clarke N., Higgins T., McArdle P., Ni Cholchuin M. and O’Gara M. (1998). J. Organometallic Chem. 559: 55
Gili P., Mederos A., Hernandez-Molina R., Dominguez S. and Nunez P. (1997). Polyhedron 16 24: 4191
Hariharan M. and Urbach F.L. (1969). Inorg. Chem. 8: 556
Batley E. and Graddon D.P. (1967). Austr. J. Chem. 20: 885
Ketelaar J.A.A., Van De Stolpe C., Goudsmit A. and Dzcubes W. (1952). Rec. Trav. Chim. 71: 1104
Ghose B.N. and Lasisi K.M. (1986). Synth. React. Inorg. Met. -Org. Chem. 16: 1121
Siddiqui R.A., Raj P. and Saxena A.K. (1996). Synth. React. Inorg. Met. -Org. Chem. 26: 1189
Lockhart T.P. and Manders W.F. (1987). J. Am. Chem. Soc. 109: 7015
Ruddick J.N.R. and Sams J.R. (1973). J. Organomet. Chem 60: 233
Sarawat S., Srivastava G.S. and Mehrotra R.C.J. (1977). Organomet. Chem. 129: 155
Sarvestani A.H. and Mohebbi S. (2006). J. Chem. Res. 4: 257
Jager E.G., Schuhmann K. and Gorls H. (1997). Inorg. Chim. Acta 255: 295
Elias H., Knoch R. and Paulus H. (1995). Inorg. Chem. 34: 4032
Taylor M.K., Reglinski J. and Wallace D. (2004). Polyhedron 23: 3201
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Asadi, M., Jamshid, K.A. & Kyanfar, A.H. Nickel(II) salophen-type complexes characterization and their thermodynamic studies with diorganotin(IV) dichlorides in chloroform. Transition Met Chem 32, 822–827 (2007). https://doi.org/10.1007/s11243-007-0272-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11243-007-0272-9