Abstract
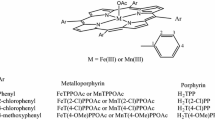
The rate of reaction of NO −2 ion with various FeIII porphyrins in the presence of PPh3 is shown to depend on the redox potential of the FeIII center. There is a linear relationship between the ease of reduction of the FeIII to FeII and the kinetics for the formation of the FeII porphyrin nitrosyl adduct, with concomitant oxidation of PPh3 to PPh3O. Cyclic voltammograms show reversible one-electron reductions that can be ascribed to the FeIII/FeII couple ranging from E1/2 = −343 to −145 mV (versus Ag/AgCl). The order of increasing half-wave reduction potentials for the FeIII/FeII porphyrin redox centers studied is octaethylporphyrin > etioporphyrin I > deuteroporphyrin IX dimethyl ester > protoporphyrin IX dimethyl ester > α,β,γ,δ-tetraphenylporphyrin. This sequence of redox potentials complements the pseudo first-order kinetics ( \({k_{\rm obs}=2.2\times10^{-3}}\) to \({13\times10^{-3}}\) m s −1) for the oxidation of PPh3 and subsequent FeII porphyrin nitrosyl adduct formation. The rates of reaction of biomimetic FeIII porphyrins with NO −2 ion demonstrate how metal center redox properties are influenced by the surrounding ligand. In this paper we have elucidated a possible mechanistic control for the rate of this reaction.
Similar content being viewed by others
References
Schwartz E. and White W.H. (1983). Trace Atmospheric Constituents, Properties, Transformation and Fates. John Wiley and Sons, New York, 1–117
Zumft W.G. (1993). Arch. Microbiol. 160: 253
R. Bonnett, A.A. Charalambides and R.A. Martin, J. Chem. Soc. Perkin. I (1977) 974
Lee D.H.K. (1970). EnvFe. Res. 3: 481
Lancaster J.R. (1992). Am. Sci. 80: 248
Vanin A.F., Mordvintcev P.I., Hauschildt S. and Mülsch A. (1993). Biochim. Biophys. Acta 1177: 37
Sharma V.S., Taylor T.G., Gardiner G.R. and Mizuka H. (1987). Biochem. 26: 3837
Finnegan M.G., Lappin A.G. and Scheidt W.R. (1990). Inorg. Chem. 29: 181
Alben J.O., Fuchsman W.H., Beaudreau C.A. and Caughey W.S. (1968). Biochemisty 7: 624
Castro C.E. and Wade R. (1985). J. Org. Chem. 50: 5342
Belser N.O. and Castro C.E. (1971). J. Agric. Food Chem. 12: 22
Castro C.E. and O’Shea S.K. (1995). J. Org. Chem. 60: 1922
O’Shea S.K., Wang W., Wade R.S. and Castro C.E. (1996). J. Org. Chem. 61: 6388
Arulsamy N., Bohle D.S., Hansert B., Powell A.K., Thomson A.J. and Wocaldo S. (1998). Inorg. Chem. 37: 746
Nasri H., Goodwin J.A. and Scheidt W.R. (1990). Inorg. Chem. 29: 185
Ellison M.E. and Scheidt W.R. (1997). J. Am. Chem. Soc. 119: 7404
Munro O.Q. and Scheidt W.R. (1998). Inorg. Chem. 37: 2308
Averill B.A. (1996). Chem. Rev. 96: 2951
Fülöp V., Moir J.W.B., Ferguson S.J. and Hajdu J. (1995). Cell 81: 369
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
O’Shea, S.K., Wall, T. & Lin, D. Activation of nitrite ion by biomimetic iron(III) complexes. Transition Met Chem 32, 514–517 (2007). https://doi.org/10.1007/s11243-007-0203-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11243-007-0203-9