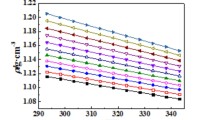
Abstract
The effect of polyvinylalcohol (PVA) on molar, Van-der Waals and the electrostriction volumes of FeCl3 and CoCl2 in pure H2O and 50% ethanol (EtOH)–water mixtures was studied. Excess volumes were also calculated and their values were discussed in terms of Pierotti theory (Scaled Particle Theory). Different theoretical energy values were calculated to explain the following parameters: volumes, dispersion, induction energies and 6-12 Lennard–Jones parameters. The increase and the decrease in excess volumes of FeCl3, CoCl2 in the absence and in the presence of polyvinyl alcohol in both aqueous and 50% EtOH–H2O mixtures were also discussed in terms of the increase or decrease of different interaction energies.
Similar content being viewed by others
References
E. Meyer and G.G. Fuller, Proceedings of the Spite, the International Society for Optical Engineering, Vol. 173, 35 (1994).
Sun Y. and Ruckenstein E. (1996). Synth. Metals 82(1): 35
Win-Pin-Chang and Wha-Tzong-Whang, Polymer, 37, 3493 (1996)
Kareiva A., Bryntse I., Karppinen M. and Niinisto I. (1996). J. Solid State Chem. 121: 356
Ikkai F., Shibayama M., Nomura S. and Han C.C. (1996). J. Polym. Sci. Part B 34: 939
K. Ito, Y. Ujihira and M. Higo, Positron Annihilation ICPA-11, 11th International Conference, Kansas City, USA (1997)
Borck J. and Osoba W. (1997). Nukleonika 42: 27
Chandar-Shekar B., Veeravazkathi V., Sakthirel S., Mangalaraj D. and Narayandass S.K. (1999). Thin Solid films 348: 122
Kweon Ho-Jin, Base Kim Gean, Sup Lim Hong, Sung Nam Sang and Park Dong Gon (1999). J. Power Sources 83: 84
King E.J. (1969). J. Phys. Chem. 73: 1220
Miller F.J. (1968). J. Phys. Chem. 72: 4589
Kim J.I. (1978). Z. Phys. Chem. N.F. 113: 129
Gomaa E.A. (1991). J. King Saud Univ. Sci. 3: 69
Wilhelm E. and Battino R. (1971). J. Chem. Phys. 75: 4012
E.A. Gomaa, Proceedings of the Kon. Ak. Van. Wet., 4, 363 (1988)
Pierotti R.A. (1976). Chem. Rev. 76: 717
Bruckl N. and Kim J.I. (1981). Z. Phys. Chem. N.F. 126: 133
Lucas M. and Feilloly A. (1970). Bull. Soc. Chim. Fr. 4: 1267
Gomaa E.A. (1985). Thermochim. Acta 91: 235
Reiss H., Frisch H.L. and Lebowiz S.L. (1957). J. Chem. Phys. 31: 369
Reiss H., Frisch H.L., Helfand E. and Lebowitz S.L. (1960). J. Chem. Phys. 32: 119
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hamada, M.A., El-Shishtawi, N.A. Influence of polyvinylalcohol on the solvation volumes of FeCl3 and CoCl2 in aqueous ethanol solutions. Transition Met Chem 32, 425–429 (2007). https://doi.org/10.1007/s11243-006-0179-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11243-006-0179-x