Abstract
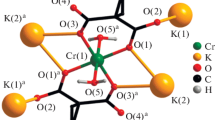
Two new chromium(III) complexes with picolinamide (pica) and oxalates, [Cr(C2O4)2(N,N′-pica)]2− and [Cr(C2O4)2(N,O-pica)]−, were obtained and the kinetics of their aquation in HClO4 solutions were studied. The aquation leads to pica liberation and proceeds in two stages: (i) the chelate-ring opening at the Cr–amide bond and (ii) the Cr–N-pyridine bond breaking, which gives free pica and cis-[Cr(C2O4)2(H2O2)2]−. In the case of N,N′-bonded pica the kinetics of both stages was determined and in the case of the N,O-bonded pica only the second stage was investigated. The following rate laws were established: (k obs)1 = k 0 + k 1 Q 1[H+] and (k obs)2 = k 2 Q 2[H+], where k 0 and k 1 are the rate constants of the chelate-ring opening in the unprotonated and protonated starting complex, and k 2 is the rate constant of the pica liberation from the protonated intermediate. Kinetic parameters are calculated and the aquation mechanism is discussed.
Similar content being viewed by others
References
J.B. Vincent (2000) Acc. Chem. Res. 33 503 Occurrence Handle1:CAS:528:DC%2BD3cXitVGnsrg%3D Occurrence Handle10.1021/ar990073r
D.D.D. Hepburn J.B. Vincent (2003) J. Inorg. Biochem. 94 86 Occurrence Handle1:CAS:528:DC%2BD3sXhs1KlsL8%3D Occurrence Handle10.1016/S0162-0134(02)00623-2
D.D.D. Hepburn J.M. Burney S.A. Woski J.B. Vincent (2003) Polyhedron 22 455 Occurrence Handle1:CAS:528:DC%2BD3sXms1eiug%3D%3D Occurrence Handle10.1016/S0277-5387(02)01369-4
S. Ogata M. Takeuchi S. Taredaria N. Yamamoto K. Iwata K. Okumura H. Taguchi (2002) Biosci. Biotechnol. Biochem. 66 641 Occurrence Handle1:CAS:528:DC%2BD38Xisl2murs%3D Occurrence Handle10.1271/bbb.66.641
H.H.G. Jellinek J.R. Urwin (1954) J. Phys. Chem. 58 548 Occurrence Handle1:CAS:528:DyaG2cXmsleksw%3D%3D Occurrence Handle10.1021/j150517a009
H.H.G. Jellinek J.R. Urwin (1954) J. Phys. Chem. 58 168 Occurrence Handle1:CAS:528:DyaG2cXjvVGgsg%3D%3D Occurrence Handle10.1021/j150512a014
H.H.G. Jellinek J.R. Urwin (1953) J. Phys. Chem. 57 900 Occurrence Handle1:CAS:528:DyaG2cXhtlGnug%3D%3D Occurrence Handle10.1021/j150510a009
R.A Olsen L. Liu N. Ghaderi A. Johns M.E. Hatcher L.J. Mueller (2003) J. Am. Chem. Soc. 125 10125 Occurrence Handle1:CAS:528:DC%2BD3sXls1ehur8%3D Occurrence Handle10.1021/ja028751j
E. Bugella-Altamirano J.M. Gonzalez-Perez D. Choquesillo-Lazarte J. Niclos-Gutierrez A. Castineiras-Campos (2000) Z. Anorg. Allg. Chem. 626 930 Occurrence Handle1:CAS:528:DC%2BD3cXisVKqtLg%3D Occurrence Handle10.1002/(SICI)1521-3749(200004)626:4<930::AID-ZAAC930>3.0.CO;2-6
M. Mad’arová, M. Sivák, L. Kuchta, J. Marek and J. Benko, J. Chem. Soc., Dalton Trans., 3313 (2004)
Q.Y. Du Y.P. Li L.Y. Xin L.M. Han (2005) Z. Crystallogr. New Cryst. Struct. 220 539 Occurrence Handle1:CAS:528:DC%2BD28XisFakt7o%3D
S.C. Chang D.Y. Park N.C. Li (1968) Inorg. Chem. 7 2144 Occurrence Handle1:CAS:528:DyaF1cXltVSktbw%3D Occurrence Handle10.1021/ic50068a039
D. Fan, C-T. Yang, J.D. Ranford and J.J. Vittal, J. Chem. Soc., Dalton Trans., 4749 (2003)
Z.N. Rocha G. Chiericato SuffixJr. E. Tfouni (1994) Inorg. Chem. 33 4619 Occurrence Handle1:CAS:528:DyaK2cXmt1Siu70%3D Occurrence Handle10.1021/ic00099a007
R.J. Balahura (1974) Inorg. Chem. 13 1350 Occurrence Handle1:CAS:528:DyaE1MXhsFOlu74%3D Occurrence Handle10.1021/ic50136a021
Inorganic Syntheses, New York, 1979, Vol. 17, p. 149
E. Kita M. Łączna (2001) Transition Met. Chem. 26 510 Occurrence Handle1:CAS:528:DC%2BD3MXlsVemtLs%3D Occurrence Handle10.1023/A:1011097613148
M. Szabłowicz E. Kita (2005) Transition Met. Chem. 30 623 Occurrence Handle10.1007/s11243-005-4588-z
M. Szabłowicz E. Kita (2006) Transition Met. Chem. 31 220 Occurrence Handle10.1007/s11243-005-6385-0
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pazderska-Szabłowicz, M., Karpiński, A. & Kita, E. New chromium(III)–picolinamide complexes. Kinetics and mechanism of picolinamide liberation in HClO4 solutions. Transition Met Chem 31, 1075–1080 (2006). https://doi.org/10.1007/s11243-006-0113-2
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s11243-006-0113-2