Abstract
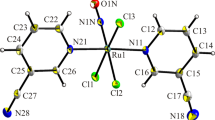
Reacting Re(CO)5Cl with the azopyridine ligand (1) (L) in boiling benzene afford the complex Re(CO)3Cl(L), (2) in excellent yield [L=2-(p-Cl-C6H4NN)C5H4N]. The chelation of the azopyridine ligand accompanied by displacement of the two carbon monoxide ligands furnish a five-membered chelate ring. Structure determination of complex (2) has revealed a distorted octahedral ReC3N2Cl coordination sphere. The Re–N(pyridine) and, Re–N(azo) distances are 2.158(3) and 2.153(6) Å respectively, and the N–N length [1.273(4) Å], implicate relatively weak Re-azo(π*) back–bonding. The Re(CO)3Cl(L) lattice consists of C–H...Cl hydrogen bonding and Cl...O non-bonded interactions constituting a supramolecular network. Extended Hückel calculations reveal that the LUMO of Re(CO)3Cl(L) is Ca. 57% azo in character. One-electron quasireversible electrochemical reduction of the complex occurs near −0.3 V versus Saturated Calomel electrode(s.c.e.) The redox orbital is believed to belong to the above noted LUMO. Electrogenerated Re(CO)3Cl(L•–) underwent spontaneous solvolytic chloride displacement in MeCN, resulting in the isolation of Re(CO)3(MeCN)(L•–). The latter complex in turn reacted with imidazole and triphenylphosphine, furnishing Re(CO)3(C3H4N2)(L•–) and Re(CO)3(PPh3)(L•–), respectively. The pattern of carbonyl stretching frequencies of these radical anion complexes is similar to that of Re(CO)3Cl(L) but with shifts to lower frequencies by 10–20 cm−1. All three radical anion systems are one-electron paramagnetic (1.7–1.8 μ B ). The unpaired electron is primarily localized on the azoheterocycle ligand in a predominantly azo-π* orbital, but a small metal contribution (185, 187Re, I=5/2) is also present. Thus Re(CO)3(MeCN)(L•–) and Re(CO)3(C3H4N2)(L•–) display six-line e.p.r. spectra (A ˜ 28 G). The line shapes and intensities are characteristic of the presence of g-strain. In the case of Re(CO)3(PPh3)(L•–) seven nearly equispaced lines are observed due to virtually equal coupling between the metal and 31P (I=½) nuclei. The g-values of the radical species are slightly higher than the free-electron value of 2.0023.
Similar content being viewed by others
References
X. Couillens, M. Gressier, M. Dartiguenave, S. Fortin and A.L. Beauchamp, J. Chem. Soc., Dalton Trans., 3032 (2002); V.M. Bereau, S.I. Khan and M.M. Abu-Omar, Inorg. Chem., 40, 6767 (2001).
J.R. Dilworth S.J. Parrott (1998) Chem. Soc. Rev. 27 43 Occurrence Handle1:CAS:528:DyaK1cXjsFOquw%3D%3D
A. Alberti and A. Hudson, J. Organometal Chem., 248, 199 (1983); G.J. Stor, F. Hartl, J.W.M. van Outersterp and D.J. Stufkens, Organometallics, 14, 1115 (1995); F.P.A. Johnson, M.W. George, F. Hartl and J.J. Turner, Organometallics, 15, 3374 (1996); H. Hartmann, T. Scheiring, J. Fiedler and W. Kaim, J. Oragnometal Chem., 604, 267 (2000); W. Kaim and S. Kohlmann, Inorg. Chem., 29, 2909 (1990); W. Kaim and S. Kohlmann, Chem. Phys. Lett., 139, 365 (1987); B.K. Panda, S. Sengupta and A. Chakravorty, J. Organometal. Chem., 689, 1780 (2004).
D.R. Striplin and G.A. Crosby, Coord. Chem. Rev., 211, 163 (2001); A.D. Guerzo, S. Leroy, F. Fages and R.H. Schmehl, Inorg. Chem., 41, 359 (2002); K. Kam-Wing Lo, W. Hui, D. Chun-Ming Ng and K. Cheung, Inorg. Chem., 41, 40 (2002); S. Sun, D.T. Tran, O.S. Odongo and A.J. Lees, Inorg. Chem., 41, 132 (2002).
G.E.D. Mullen P.J. Blower D.J. Price A.K. Powell M.J. Howard M.J. Went (2000) Inorg. Chem. 39 4093 Occurrence Handle10.1021/ic991240m Occurrence Handle1:CAS:528:DC%2BD3cXls1Ors7Y%3D Occurrence Handle11198864
T.J. Burchell, D.J. Eisler and R.J. Puddephatt, Inorg. Chem.,43, 5550 (2004); D. Vujovic, H.G. Raubenheimer and L.R. Nassimbeni, J. Chem. Soc., Dalton Trans., 631 (2003); A. Dey, R.K.R. Jetti, R. Boese and G.R. Desiraju, Cryst. Eng. Comm., 5, 248 (2003).
P. Bishop, P. Marsh, A.K. Brisdon, B.J. Brisdon and M.F. Mahon, J. Chem. Soc., Dalton Trans., 675 (1998); Z. Qin, M.C. Jennings, R.J. Puddephatt and K.W. Muir, CrystEngComm., 11, 73 (2000); I.D.E.T. Wilton-Ely, A. Schier, N.W. Mitzel and H. Schmidbaur, J. Chem. Soc., Dalton Trans., 1058 (2001).
S. Banerjee S. Bhattacharyya B.K. Dirghangi M. Menon A. Chakravorty (2000) Inorg. Chem. 39 6 Occurrence Handle10.1021/ic990465u Occurrence Handle1:CAS:528:DyaK1MXnvFKgsbc%3D Occurrence Handle11229035
I. Chakraborty, S. Sengupta, S. Das, S. Banerjee and A. Chakravorty, Dalton Trans., 134 (2003).
I. Chakraborty, S. Bhattacharyya, S. Banerjee, B.K. Dirghangi and A. Chakravorty, J. Chem. Soc., Dalton Trans., 3747 (1999); S. Bhattacharyya, I. Chakraborty, B.K. Dirghangi and A. Chakravorty, J. Chem. Soc., Chem. Comm., 1813 (2000); S. Das, I. Chakraborty and A. Chakravorty, Polyhedron, 22, 901 (2003); S. Sengupta, I. Chakraborty and A. Chakravorty, Eur. J. Inorg. Chem., 1157 (2003).
N. Campbell, A.W. Henderson and D. Tayler, J. Chem. Soc., 1281 (1953).
G.K. Lahiri, S. Bhattacharya, B.K. Ghosh and A. Chakravorty, Inorg. Chem., 26, 4324 (1987); D.T. Sawyer and J.L. Roberts, Jr., Experimental Electrochemistry for Chemists, Wiley, New York, 1974, vol. 167.
A.C.T. North D.C. Phillips F.A. Mathews (1968) Acta Crystallogr. Sect. A 24 351 Occurrence Handle10.1107/S0567739468000707
G.M. Sheldrick, SHELXTL, version 5.03; Siemens Analytical X-ray System Madison, WI, (1994)
C.L. Gibb E.D. Stevens B.C. Gibb (2001) J. Am. Chem. Soc. 123 5849 Occurrence Handle10.1021/ja005931p Occurrence Handle1:CAS:528:DC%2BD3MXjvVWhsrw%3D Occurrence Handle11403638
C. Mealli D.M. Proserpio (1990) J. Chem. Edu. 67 399 Occurrence Handle1:CAS:528:DyaK3cXksFyjtLs%3D
J.R. Pilbrow (1990) Transition ion Paramagnetic Resonance Clarendon Press Oxford
M. Shivakumar, K. Pramanik, P. Ghosh and A. Chakravorty, Chem. Comm., 2103 (1998); M. Shivakumar, K. Pramanik, P. Ghosh and A. Chakravorty, Inorg. Chem., 37, 5968 (1998); M. Shivakumar, K. Pramanik, I. Bhattacharyya and A. Chakravorty, Inorg. Chem., 39, 4332 (2000).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Sengupta, S., Panda, B.K. Synthesis and structure of the 2-(chlorophenylazo)pyridine chelated tricarbonylrhenium(I) complex and its anion radical derivatives via electrochemical reduction. Transition Met Chem 30, 426–432 (2005). https://doi.org/10.1007/s11243-005-0726-x
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s11243-005-0726-x