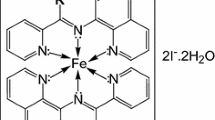
Abstract
Complexes of FeII with monoxime and dioxime ligands have been isolated and characterised. Kinetic results and rate laws are reported for acid aquation and base hydrolysis of these complexes in H2O and in MeOH–H2O mixtures. Kinetics of acid catalysed aquation of FeII–monoxime complexes follow a rate law with kobs = k2[H+] + k3[H+]2, while kinetics of acid dissociation and base hydrolysis of the FeII–dioxime complex follow rate laws with kobs = k2[H+] and kobs = k2[OH−]. Acid aquation and base hydrolysis mechanisms are proposed. The solubilities of FeII–monoxime and –dioxime complex salts are reported and transfer chemical potentials of their complex cations are calculated. Solvent effects on reactivity trends have been analysed into initial and transition state components. These are determined from transfer chemical potentials of reactant and kinetic data. Rate constant trends from these complexes are compared and discussed in terms of ligand structure and solvation properties. Our kinetic results give information relevant to the application of these ligands as analytical reagents for trace FeII in acidic and neutral media, in water and in aqueous alcohols.
Similar content being viewed by others
References
R.K. Murmann E.A. Healy (1961) J. Am. Chem. Soc. 83 2092
L.F. Lindoy S.E. Livingston (1967) Coord. Chem. Rev. 2 173
P. Krumholz (1971) Struct. Bonding. 9 139
A.A Schilt (1969) Analytical Applications of 1,10-Phenanthroline and Related Compounds Pergamon Oxford.
Hanania G.I.H., Irvine D.H. (1962) J. Chem. Soc., 2745.
Hanania G.I.H., Irvine D.H., Shurayh F. J. (1965). Chem. Soc., 1149.
H. Hartkamp (1960) Fresenius Z. Anal. Chem. 19 178
E.I. Baucom R.S. Drago (1971) J. Am. Chem. Soc. 93 6469
M. Satake N. Sugita M. Katyal (1990) Ann. Chim. 80 385
M.J. Blandamer J. Burgess B. Clark P.P. Duce A.W. Hakin N. Gosal S. Radulović P. Guardado F. Sanchez C.D. Hubbard E.A. Abu-Gharib (1986) J. Chem. Soc., Faraday Trans. 1 IssueID82 1471
D.W. Margerum. J. Am. Chem. Soc. 79: 2728 (1957); J. Burgess R.H. Prince. J. Chem. Soc., 4697, (1965).
T.N. Sorrell (1997) Organic Chemistry University Science Books Sausalito
Lipschutz M.M. Schaum’s Outline of Theory and Problems of Differential Geometry, McGraw-Hill, New York, 1969; P.K. Jain and K. Ahmad. Analytical Geometry of Two Dimensions, 2nd edit., Wiley Eastern, India 1986.
E.A. Abu-Gharib J. Burgess. Transition Met. Chem. 18: 623 (1993).
M.L. Tobe J. Burgess. Inorganic Reaction Mechanisms, Addison–Wesley–Longman, Harlow, 1999.
J.D. Miller W.R. McWhinnie. Adv. Inorg. Chem. Radiochem. 12: 135 (1969).
M.J. Blandamer J. Burgess. Coord. Chem. Rev. 31: 93 (1980).
J. Burgess M.V. Twigg. J. Chem. Soc. Dalton Trans., 2032 (1974).
G.K. Pagenkopf D.W. Margerum (1968) Inorg. Chem. 7 2514
F.H. Fraser P. Esptein D.L. Macero (1972) Inorg Chem. 11 2031
Blandamer MJ., Burgess J., Haines RI., Mekhail F.M., Askalani P. J. Chem. Soc., Dalton Trans., 1001 (1978).
I.L. Finar (1973) Organic Chemistry, Vol. 1 EditionNumber6 Longman London.
H. Hartkamp. Naturwissenschaften. 45: 211 (1958); H. Hartkamp. Z. Anal. Chem. 170: 399 (1959).
S. Bolton (1963) J. Pharm. Sci. 52 858 Occurrence Handle14061045
D.K. Banerjea K.K. Tripathi (1960) Analyt. Chem. 32 1196
B. Sen. Chem. Ind. (London), 562 (1958); F. Trusell H. Diehl. Analyt. Chem. 31: 1978 (1959).
Serpone N., Ponterini G., Jamieson MA., F. Bolletta M.M. Maestri. Coord. Chem. Rev. 50: 209 (1983); Gillard RD., D.W. Knight P.A. Williams. Transition Met. Chem. 5: 321 (1980); R.D. Gillard. Coord. Chem. Rev. 50: 303 (1983); E.C. Constable. Polyhedron. 2: 551 (1983).
R.D. Gillard. Coord. Chem. Rev. 16: 67 (1975); Blandamer MJ., J. Burgess E.A. Abu-Gharib. Transition Met. Chem. 9: 193 (1984).
Sidahmed I.M., Wells C.F. J. Chem. Soc., Dalton Trans.. 10: 2034 (1981); J. Burgess E.A. Abu-Gharib. Transition Met. Chem. 9: 234 (1984); A.A. El-Samahy, E.A. Abu-Gharib, A. Eltaher, R.M. El-Khatib J. Burgess. Transition Met. Chem. 17: 438 (1992).
MH. Abraham T. Hill HC. Ling R.A. Schulz R.A.C. Watt (1984) J. Chem. Soc., Faraday Trans 1 IssueID80 489
A. Al Alousy J. Burgess (1987) Transition Met. Chem. 12 565
S. Alshehri MJ. Blandamer J. Burgess P. Guardado C.D. Hubbard (1993) Polyhedron. 12 445
Krumholz P. Inorg. Chem. 9: 609 (1965); J. Burgess. J. Chem. Soc. (A), 497 (1968).
A. Desoky (2003) MSc. Thesis. South Valley University Sohag Egypt
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Abu-Gharib, EE.A., Komy, Z., Eltaher, AE. et al. Kinetic, solvation and reactivity studies of iron(II) complexes of monoxime and dioxime ligands. Transition Met Chem 30, 357–366 (2005). https://doi.org/10.1007/s11243-004-6967-2
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s11243-004-6967-2