Abstract
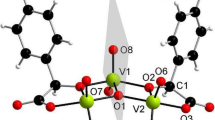
Peroxovanadium(V) complexes of α-hydroxyhippuric acid (α-H2hhip), M 2[V2O2(O2)2(α-hhip)2]·nH2O, [M=K+ (1), NH4 + (2), NEt 4 + (3), NBu 4 + (4); n=5.5, 3, 8, 5) have been prepared and characterized by elemental analysis, i.r., u.v.–vis. and 51V-n.m.r. spectroscopies and by thermal analysis. The X-ray structure determination of (4) revealed the presence of dinuclear [V2O2(O2)2(R-α-hhip)(S-α-hhip)]2− anions with a planar V2O2 bridging core and seven-coordinated central atoms. The coordination geometry of the vanadium atom is a distorted pentagonal bipyramid.
Similar content being viewed by others
References
A.S. Tracey and D.C. Crans, (Eds.), Vanadium Compounds: Chemistry, Biochemistry and Therapeutic Applications, ACS Symposium Series 711, ACS Books, Washington, 1998.
H. Sigel and A. Sigel, (Eds.), Vanadium and its Role in Life. Metal Ions in Biological Systems, Marcel Dekker, New York, Basel, Hong Kong, 1995.
R. Wever and K. Kustin, in A.G. Sykes, (Ed.), Advances in Inorganic Chemistry, Vanadium a Biologically Relevant Element, Academic Press, New York, 1990, p. 103.
J. Liang, M. Madden, V.K. Shah and R.H. Burris, Biochemistry, 29, 8577 (1990).
M. Weyand, H. Hecht, M. Kiess, M. Liaud, H. Vilter and D. Schomburg, J. Mol. Biol., 293, 595 (1999).
H. Vilter, in H. Sigel and A. Sigel, (Eds.), Metal Ions in Biological Systems, Vol. 31, Vanadium and its Role in Life, Marcel Dekker, New York, 1995, p. 325.
A. Butler, Curr. Opin. Chem. Biol., 2, 279 (1998).
E. Bayer, in H. Sigel and A. Sigel, (Eds.), Metal Ions in Biological Systems, Vol. 31, Amavadin, the Vanadium Compound of Amanitae, Marcel Dekker, New York, 1995, p. 407.
M. Smith, D.E. Ryan, K. Nakanishi, P. Frank and K.O. Hodgson, in H. Sigel and A. Sigel, (Eds.), Metal Ions in Biological Systems, Vol. 31, Vanadium in Ascidians and the Chemistry in Tunichromes, Marcel Dekker, New York, 1995, p. 423.
J.J.R. Frausto da Silva, Chem. Speciation Bioavailability, 1, 139 (1989).
E. de Boer, Y. van Kooyk, M.G.M. Tromp, H. Plat and R. Wever, Biochim. Biophys. Acta, 869, 48 (1986).
H. Plat, B.E. Krenn and R. Wever, J. Biochem., 248, 277 (1987).
A. Butler, M.J. Clague and G.E. Meister, Chem. Rev., 94, 625 (1994).
S. Kadota, I.G. Fantus, G. Deragon, H.J. Guyda, B. Hersh and B.I. Posner, Biochem. Biophys. Res. Commun., 147, 259 (1987).
D. Heffetz, I. Bushkin, R. Dror and Y. Zick, J. Biol. Chem., 565, 2896 (1990).
C. Orvig, K.H. Thompson, M. Battell and J.H. McNeill, in H. Sigel and A. Sigel, (Eds.), Vanadium Compounds as Insulin Mimetics, Metal Ions in Biological Systems, Vol. 31, Vanadium and its Role in Life, Marcel Dekker, New York, 1995, p. 575.
K. Hor, O. Gimple, P. Schreier and H.-U. Humpf, J. Org. Chem., 63, 322 (1998).
M.H. Hyun, M.H. Kang and S.C. Han, Tetrahedron Lett., 40, 3435 (1999).
S.V. Pansare, R.G. Ravi and R.P. Jain, J. Org. Chem., 63, 4120 (1998).
W. Adam, M. Lazarus, B. Boss, C.R. Saha-Moller, H.-U. Humpf and P. Schreier, J. Org. Chem., 62, 7841 (1997).
K.C. Nicolau and R.K. Guy, Angew. Chem., Int. Ed. Engl., 34, 2079 (1995).
S. Omura and H. Tanaka, in S. Omura, (Ed.), Macrolide Antibiotics: Chemistry, Biology and Practice, Academic Press, New York, 1984, p. 351.
P. Schwendt, P. Švančárek, I. Smatanová and J. Marek, J. Inorg. Biochem., 80, 59 (2000).
C. Djordjevic, M. Lee and F. Sinn, Inorg. Chem., 28, 719 (1989) and references cited therein.
C. Djordjevic, M. Lee-Renslo and E. Sinn, Inorg. Chim. Acta, 233, 97 (1995).
P. Schwendt, P. Švančárek, L'. Kuchta and J. Marek, Polyhedron, 17, 2161 (1998).
I. Kutá-Smatanová, J. Marek, P. Švančárek and P. Schwendt, Acta Cryst. C, 56, 154 (2000).
F. Demartin, M. Biagioli, L. Strinna-Erre, A. Panzanelli and G. Micera, Inorg. Chim. Acta, 299, 123 (2000).
P. Švančárek, P. Schwendt, J. Tatiersky, I. Smatanová and J. Marek, Monatsh. Chem., 131, 145 (2000).
P. Sgarabotto, F. Bisceglie, G. Pelosi and L. Abdel-Rahman, Polyhedron, 18, 2505 (1999).
K. Poon and K.S. Pang, Drug Metab. Dispos., 23, 255 (1995).
C. Zuppi, D. Valeria Rossetti, A. Vitali, F. Vincenzoni, B. Giardina, M. Castagnola and I. Messana, J. Chromatogr. B., 793, 223 (2003).
P. Schwendt, M. Ahmed and J. Marek, Inorg. Chem. Commun., 7, 631 (2004).
G.M. Sheldrick, SHELX-97 Program, Dept. Inorg. Chem., University of Göttingen, Germany.
K. Nakamoto, Infrared and Raman Spectra of Inorganic and Coordination compounds, 4th edit., Wiley and Sons, New York, Chichester, Brisbane, Toronto, Singapore, 1990, p. 253.
H. Mimoun, L. Saussine, E. Daire, M. Postel, J. Fischer and R. Weiss, J. Am. Chem. Soc., 105, 3101 (1983).
P. Schwendt, Coll. Czech. Chem. Commun., 48, 248 (1983).
C.K. Johnson and M.N. Burnett, ORTEP-III: Oak Ridge Thermal Ellipsoid Plot Program for Crystal Structure Illustrations, Report ORNL-6895, National Laboratory, Tenn, 1996.
M. Orhanovic and R.G. Wilkins, J. Am. Chem. Soc., 89, 278 (1967).
M. Sivák, Chem. Papers, 41, 311 (1987).
V. Conte, F. Di Furia and S. Moro, J. Mol. Catal., 104, 159 (1995).
S. Hati, R.J. Batchelor, F.W.B. Einstein and A.S. Tracey, Inorg. Chem., 40, 6258 (2001).
L.L.G. Justino, M.L. Ramos, M.M. Caldeira and V.M.S. Gil,Inorg. Chim. Acta, 311, 119 (2000).
L.L.G. Justino, M.L. Ramos, M.M. Caldeira and V.M.S. Gil, Eur. J. Inorg. Chem., 1617 (2000).
A. Gorzsás, I. Andersson and L. Pettersson, Dalton Trans., 2503 (2003).
M. Ahmed, P. Schwendt, J. Marek and M. Sivák, Polyhedron, 23, 655 (2004).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Ahmed, M., Schwendt, P., Sivá, M. et al. Synthesis and characterization of vanadium(V) complexes with α-hydroxyhippuric acid. The X-ray crystal structure of (NBu 4)2[V2O2(O2)2(R-α-hhip) (S-α-hhip)]·5H2O,[α-hhip = α-hydroxyhippurato(2-)]. Transition Metal Chemistry 29, 675–680 (2004). https://doi.org/10.1007/s11243-004-5363-2
Issue Date:
DOI: https://doi.org/10.1007/s11243-004-5363-2