Abstract
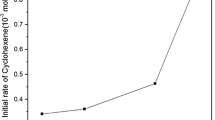
The reactions between substituted benzyl alcohols were found to proceed through ester formation. The ester thus formed decomposes in a slow step to produce chromium(IV). Since our oxidant was supported on a polymeric material the intermediate chromium(IV) will further oxidize another molecule of alcohol generating a free radical in a fast step. The free radical subsequently reacts with another oxidant site in the polymeric reagent in a fast step leading to the formation of chromium(V). The intermediate chromium(V) in the last step reacts with alcohol to produce an aldehyde. The activation parameters were also determined and the mechanism is predicted.
Similar content being viewed by others
References
I. Nongknrich M.K. Mahanti (1966) Bull. Chem. Soc. Jpn. 69 1403
I. Nongknrich M.K. Mahanti (1995) Bull Chem. Soc. Jpn. 68 3325
Pitre S.V., M.V., Ram Reddy Y.D. (1997) Vankar; J. Chem. Res (S)., 462.
R.C. Larock I Fleming (1991) Comprehensive Organic Synthesis Pergamon Press Oxford
(a) Mancuso A., Huang S.L., Swern D., J. Org. Chem., 43, 2480 (1978); (b) Hirano M., Kurodo H., T. Morimoto Bull. Chem. Soc. Jpn., 63, 2433 (1990); (c) Lou W.X., Lou J.D., Synth. Commun., 767 (1992).
D.B. Dess J.C. Martin (1983) J. Org. Chem. 48 4155 Occurrence Handle10.1021/jo00170a070
Hutchins R.O., Natale N.R., Cook W.J., (1977) Tetrahedron Lett., 4167.
Geethakumari K., Sreekumar K., J. Appl. Polym Sci., 67, 799.
G. Cainelii G. Cardillo M. Orena S. Sardri (1976) J. Am. Chem. Soc. 98 6737 Occurrence Handle10.1021/ja00437a071
T. Brunelet C. Jouitteau G. Gelhard (1986) J. Org. Chem. 51 4016 Occurrence Handle10.1021/jo00371a019
A.J. Buglas S. John Waterhouse (1987) J. Chem. Edu. 64 3712
R.W. Fulmer (1962) J. Org. Chem. 27 4115
(a) Sevcik S., Stamberg J.,, Prochazka M., Coll. Czech. Chem. Comm., 33, 1327 (1968). (b) Tartarelli R., Lucchesi A., Stappato B., Catalysis J., 19, 310 (1970).
J.Y. Tong E.L. King (1960) J. Am. Chem. Soc. 82 3805 Occurrence Handle10.1021/ja01500a001
F.H. Westheimer (1949) Chem. Rev. 45 419 Occurrence Handle10.1021/cr60142a002
J. Rocek A.E. Radkowsky (1968) J. Am. Chem. Soc. 90 2986 Occurrence Handle10.1021/ja01013a059
J.H. Espenson (1964) J. Am. Chem. Soc. 86 5101 Occurrence Handle10.1021/ja01077a012
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Jawanjal, A.L., Hilage, N.P. Kinetics and mechanism of oxidation of substituted benzyl alcohols by polymer supported chromic acid. Transition Met Chem 30, 290–293 (2005). https://doi.org/10.1007/s11243-004-4055-2
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s11243-004-4055-2