Abstract
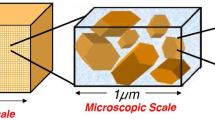
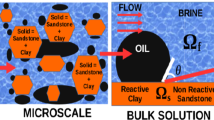
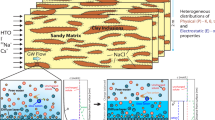
The electroremediation process is an efficient method for removing pollutants from clayey soils. We model this process in kaolinite clays considering three scales—nano, micro and macro—under the assumption of a stratified geometry in conjunction with more realistic Danckwerts’ boundary conditions imposed at the electrodes. The resulting multiscale model is a coupled system of nonlinear partial differential equations. We derive analytical solutions of the macroscopic equations considering the asymptotic behavior of strongly convective and diffusive regimes. We perform numerical simulations of different scenarios for the electroremediation using the Galerkin finite element method together with a staggered algorithm and the Newton–Raphson method. We validate the accuracy of the proposed algorithm by comparing the discrete solutions to the analytical ones. Finally, we explore and discuss optimal scenarios for the electroremediation process depending on the input values of pH, electrical current, and mass inflow using dimensionless numbers defined from the analytical solutions.











Similar content being viewed by others
Abbreviations
- \(\text{ Na }^+\), \(\text{ H }^+\), \(\text{ Cl }^-\), \(\text{ OH }^-\) :
-
Ionic species
- p :
-
Thermodynamic pressure (Pa)
- x, y :
-
Parallel and orthogonal coordinates (m)
- \(C_{\mathrm{i}_\mathrm{b}}\) :
-
Ionic bulk concentration with \(i=\hbox {Na}^+,\hbox {H}^+,\hbox {Cl}^-,\hbox {OH}^-\) (\(\hbox {mol}/\hbox {m}^3\))
- \(C_{\mathrm{b}}\) :
-
Bulk concentration (\(\hbox {mol}/\hbox {m}^3\))
- F :
-
Faraday constant (\(\hbox {C}/\hbox {mol}\))
- H :
-
Half distance between the particles (m)
- K :
-
Equilibrium constant (l/mol)
- \(K_\mathrm{w}\) :
-
Ionic product of water (\((\hbox {mol}/\hbox {m}^3)^2\))
- \(L_\mathrm{D}\) :
-
Debyes length (m)
- L :
-
Macroscopic characteristic length (m)
- l :
-
Microscopic characteristic length (m)
- M :
-
Metallic ions at the surface
- \(I_0\) :
-
Current imposed at the anode (\(\hbox {A}/\hbox {m}^2\))
- \(C_\mathrm{{Na}}^\mathrm{{res}}\) :
-
Salt concentration imposed at the reservoir (\(\hbox {mol}/\hbox {m}^3\))
- R :
-
Ideal gas constant (J/(molK))
- T :
-
Temperature (K)
- \(\dot{m}\) :
-
Mass production due to hydrolysis
- Y :
-
Cell domain
- \(Y_\mathrm{f},Y_\mathrm{s}\) :
-
Fluid and solid subdomains
- \(\mathbf {x}\) :
-
Macroscopic coordinate (m)
- \(\mathbf {y}\) :
-
Microscopic coordinate (m)
- \(\mathbf {f}\) :
-
Characteristic function
- \(\mathbf {A}^{\mathbf {eff}}\) :
-
First Onsager coefficient (\((\hbox {cm}^2)/(\hbox {mol})\))
- \(\mathbf {B}^{\mathbf {eff}}\) :
-
Second Onsager coefficient (\((\hbox {cm}^2)/(\hbox {mol})\))
- \(\mathbf {C}^{\mathbf {eff}}\) :
-
Effective electric conductivity (\(\hbox {C}/(\hbox {ms})\))
- \(\widehat{\mathbf {D}}^{\mathbf {eff}}\) :
-
Net effective diffusivity \(\text{ H }^+-\hbox {OH}^-\) (\(\hbox {m}^2/\hbox {s}\))
- \(\mathbf {D}_{\mathbf{Na}^{+}}^{\mathbf {eff}}\) :
-
Effective sodium diffusivity (\(\hbox {m}^2/\hbox {s}\))
- \(\mathbf {K}_{\mathbf{P}}^{\mathbf {eff}}\) :
-
Hydraulic conductivity [\(\hbox {m}^2/(\hbox {Pas})\)]
- \(\mathbf {K}_{\mathbf{E}}^{\mathbf {eff}}\) :
-
Electroosmotic permeability [\(\hbox {m}/(\hbox {Vs})\)]
- \(\mathbf {I}_{\mathbf{f}}^{\mathbf {eff}}\) :
-
Effective electric current [\(\hbox {C}/(\hbox {m}^2\,\hbox {s})\)]
- \(\mathbf {J}^{\mathbf {eff}}\) :
-
Effective ionic flux [\(\hbox {mol}/(\hbox {m}^2\,\hbox {s})\)]
- \(\mathbf {V}_{\mathbf {D}}\) :
-
Darcys velocity (m/s)
- \(\nabla _{x}\), \(\nabla _{y}\) :
-
Spatial gradients
- \(\delta \) :
-
Half particle thickness (m)
- \(\varepsilon \) :
-
Perturbation parameter
- \(\widetilde{\varepsilon }_{0}\) :
-
Permittivity of the free space [C/(Vm)]
- \(\widetilde{\varepsilon }_{\mathrm{r}}\) :
-
Dielectric constant
- \(\phi \) :
-
Macroscopic electric potential (V)
- \(\varphi \) :
-
EDL electric potential (V)
- \(\overline{\phi }\) :
-
Dimensionless macroscopic electric potential
- \(\eta \) :
-
Porosity
- \( \varvec{\kappa _{P}}\) :
-
Characteristic tensorial function
- \(\mu _\mathrm{f}\) :
-
Viscosity of the water (Pas)
- \(\pi _{\mathrm{P}}\) :
-
Microscopic pressure
- \(\sigma \) :
-
Surface charge density (\(\hbox {C}/\hbox {m}^2\))
- \(\zeta \) :
-
Zeta potential (V)
- \(\overline{\zeta }\) :
-
Dimensionless zeta potential
- \({\varTheta }\) :
-
Nonlinear parameter in the advection term of the protons
- \({\varGamma }_{\mathrm{MAX}}\) :
-
Density of available sites (\(\hbox {sites}/\hbox {nm}^2\))
- \({\varOmega }^\mathrm{m}\), \({\varOmega }\) :
-
Microscopic and Macroscopic domain
- \({\varOmega }_\mathrm{s}\),\({\varOmega }_\mathrm{f}\) :
-
Solid and fluid microscopic domain
- \({\varGamma }_{\mathrm{fs}}\) :
-
Solid–fluid interface
References
Acar, Y.B., Alshawabkeh, A.N.: Principles of electrokinetics remediation. Environ. Sci. Technol. 27, 2638–2647 (1993)
Acar, Y.B., Alshawabkeh, A.N., Gales, J.: Fundamentals of extracting species from soils by electrokinetics. Waste Manag. (Oxford) 13, 141–151 (1993)
Alshawabkeh, A.N., Acar, Y.B.: Electrokinetic remediation: theoretical model. J. Geotech. Eng. 122, 186–196 (1996)
Auriault, J., Boutin, C., Geindreau, C.: Homogenization of coupled phenomena in heterogenous media. Wiley, Hoboken (2009)
Beddiar, K., Fen-Chong, T., Dupas, A., Berthaud, Y., Dangla, P.: Role of ph in electro-osmosis: experimental study on nacl-water saturated kaolinite. Transp. Porous Media 61(1), 93–107 (2005)
Brooks, A.N., Hughes, T.J.R.: Streamline Upwind Petrov–Galerkin formulations for convection dominated flows with particular emphasis on the incompressible Navier-Stokes equations. Comput. Methods Appl. Mech. Eng. 32, 199–259 (1982)
Coelho, D., Shapiro, M., Thovert, J., Adler, P.: Electrokinetic phenomena in porous media. J. Colloid Interface Sci. 181, 169–190 (1996)
Danckwerts, P.V.: Continuous flow systems; distribution of residence times. Chem. Eng. Sci. 2, 1–13 (1953)
Dormieux, L., Barboux, P., Coussy, O., Dangla, P.: A macroscopic model of the swelling phenomenon of a saturated clay. Eur. J. Mech. Solids 14(6), 981–1004 (1995)
Edwards, D.: Charge transport through a spatially periodic porous medium: electrokinetic and convective dispersion phenomena. Philos. Trans. R. Soc. Lond. A353, 174–180 (1995)
Gupta, A., Coelho, D., Adler, P.: Universal electro-osmosis for porous media. J. Colloid Interface Sci. 319, 549–554 (2008)
Huertas, F., Chou, L., Wollast, R.: Mechanism of kaolinite dissolution at room temperature an in pressure: part 1. Surface speciation. Geochim. Cosmochim. Acta 62, 417–431 (1997)
Hunter, R.: Zeta Potential in Colloid Science: Principles and Applications. Academic Press, Cambridge (1981)
Hunter, R.: Introduction to Modern Colloid Science. Oxford University Press, Oxford (1994)
Kim, J., Tchelepi, H.A., Juanes, R.: Stability and convergence of sequential methods for coupled flow and geomechanics: Drained and undrained splits. Comput. Methods Appl. Mech. Eng. 200, 20942116 (2011)
Landau, L., Lifshitz, E.: Electrodynamics of Continuous Media. Pergamon Press, Oxford (1960)
Le, T., Moyne, C., Murad, M., Lima, S.: A two-scale non-local model of swelling porous media incorporating ion size correlation effects. J. Mech. Phys. Solids 61, 2493–2521 (2013)
Lemaire, T., Moyne, C., Stemmelen, D.: Modelling of electro-osmosis in clayey materials including ph effects. Phys. Chem. Earth 32, 441–452 (2007)
Lima, S., Murad, M.A., Moyne, C., Stemmelen, D.: A three scale model of ph-dependent steady flows in 1:1 clays. Acta Geotech. 3, 153–174 (2008)
Lima, S., Murad, M.A., Moyne, C., Stemmelen, D.: A three scale model of ph-dependent flow and ion transport with equilibrium adsorption in kaolinite clays: I homogenization analysis. Transp. Porous Media 85, 23–44 (2010a)
Lima, S., Murad, M.A., Moyne, C., Stemmelen, D.: A three scale model of ph-dependent flow and ion transport with equilibrium adsorption in kaolinite clays: Ii effective-medium behavior. Transp. Porous Media 85, 45–78 (2010b)
Mainka, J., Murad, M.A., Moyne, C., Lima, S.A.: A modified effective stress principle for unsaturated swelling clays derived from microstructure. Vadose Zone J. 13, 1–17 (2014)
Malevich, A.E., Mityushev, V.V., Adler, P.: Electrokinetic phenomena in wavy channels. J. Colloid Interface Sci. 345, 72–87 (2010)
Moyne, C., Murad, M.: A two-scale model for coupled electro-chemo-mechanical phenomena and onsager s reciprocity relations in expansive clays: I homogenization analysis. Transp. Porous Media 62, 333–380 (2006)
Murad, M.A., Moyne, C.: A dual-porosity model for ionic solute transport in expansive clays. Comput. Geosci. 12, 47–82 (2008)
Olphen, V.: An Introduction to Clay Colloid Chemistry: For Clay Technologists, Geologists, and Soil Scientists. Wiley, New York (1977)
Ponce, R., Murad, M.A., Lima, S.A.: A two-scale computational model of ph-sensitive expansive porous media. J. Appl. Mech. 80, 20,903–20,917 (2013)
Reddy, K.R., Cameselle, C.: Electrochemical Remediation Technologies for Polluted Soils, Sediments and Ground- water. Wiley, Hoboken (2009)
Samson, E., Marchand, J., Robert, J.L., Bournazel, J.P.: Modelling ion diffusion mechanisms in porous media. Int. J. Numer. Meth. Eng. 46, 2043–2060 (1999)
Sanchez-Palencia, E.: Non-Homogeneous Media and Vibration Theory. Lectures Notes in Physics (1980)
Acknowledgements
The authors would like to acknowledge professors Marcio Arab Murad and Claude Boutin for their technical support.
Author information
Authors and Affiliations
Corresponding author
Appendix: A Weak Formulation Linearized
Appendix: A Weak Formulation Linearized
The variational model given by (46), (47) and (48) is now linearized by Newton–Raphson method. To this end, we introduce the nonlinear objective functions \(F(C^n_{\mathrm{Na}_\mathrm{b}^+})\), \(F(C^n_\mathrm{{H}_\mathrm{b}^+})\) e \(F(\phi ^n)\), given by
Defining \({\varUpsilon }^n := \{C^n_{\mathrm{Na}_\mathrm{b}}, C^n_\mathrm{{H}_\mathrm{b}}, \phi ^n\}\) the triple unknown of the system (46)–(48) and the function \(F({\varUpsilon }^n) := F(C^n_{\mathrm{Na}_\mathrm{b}}, C^n_\mathrm{{H}_\mathrm{b}}, \phi ^n)\), denoting k the Newton–Raphson method iteration index and expanding \(F({\varUpsilon }^n)\) in Taylor series around to the point \({\varUpsilon }^{k,n}\) near the root we have
Imposing the condition \(F({\varUpsilon }^{k+1,n}) = 0\), and neglecting the second order terms, we obtain the expression of Newton–Raphson method:
where \(F'({\varUpsilon }^{k,n})\) is the jacobian of the objective function.
The Eq. (54) is used recursively from an initial estimate \({\varUpsilon }^{0,n}\) to the problem root to obtain a sequence values of \({\varUpsilon }^{k+1,n}\) that approximate the nonlinear problem solution. The stopping criterion of the iterative process consists of defining a acceptable value for tolerance \(\varepsilon \), so that
Rights and permissions
About this article
Cite this article
Igreja, I., Lima, S.A. & Klein, V. Asymptotic Analysis of Three-Scale Model of pH-Dependent Flows in 1:1 Clays with Danckwerts’ Boundary Conditions. Transp Porous Med 119, 425–450 (2017). https://doi.org/10.1007/s11242-017-0891-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11242-017-0891-3