Abstract
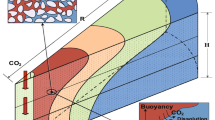
During CO2 injection into brine aquifers-containing residual and/or dissolved CH4, three distinct regions develop: (1) a single-phase, dry-out region around the well-bore filled with pure supercritical CO2; (2) a two-phase, two-component system containing CO2 and brine; and (3) a two-phase, two-component system containing CH4, and brine. This article extends an existing analytical solution, for pressure buildup during CO2 injection into brine aquifers, by incorporating dissolved and/or residual CH4. In this way, the solution additionally accounts for partial miscibility of the CO2–CH4–brine system and the relative permeability hysteresis associated with historic imbibition of brine and current drainage due to CO2 injection and CH4 bank development. Comparison of the analytical solution results with commercial simulator, CMG-GEM, shows excellent agreement among a range of different scenarios. The presence of residual CH4 in a brine aquifer summons two competing phenomena, (1) reduction in relative permeability (phase interference), which increases pressure buildup by reducing total mobility, and (2) increase in bulk compressibility which decreases pressure buildup of the system. If initial CH4 is dissolved (no free CH4), these effects are not as important as they are in the residual gas scenario. Relative permeability hysteresis increased the CH4 bank length (compared to non-hysteretic relative permeability), which led to further reduction in pressure buildup. The nature of relative permeability functions controls whether residual CH4 is beneficial or disadvantageous to CO2 storage capacity and injectivity in a candid brine aquifer.
Similar content being viewed by others
Abbreviations
- α :
-
Dimensionless compressibility parameter [−]
- μ a :
-
Dynamic viscosities of brine with dissolved CO2 [ML−1T−1]
- μ a :
-
Dynamic viscosity of the aqueous phase [ML−1T−1]
- μ b :
-
Dynamic viscosities of brine with dissolved CH4 [ML−1T−1]
- μ c :
-
Dynamic viscosities of water-free CO2 [ML−1T−1]
- μ g :
-
Dynamic viscosities of CO2-rich gas [ML−1T−1]
- μ g :
-
Dynamic viscosity of the gas phase [ML−1T−1]
- μ m :
-
Dynamic viscosities of CH4-rich gas [ML−1T−1]
- ω ca :
-
Mass fractions of CO2 in the aqueous phase [−]
- ω cg :
-
Mass fractions of CO2 in the gas phase [−]
- ω ma :
-
Mass fractions of CH4 in the aqueous phase [−]
- ω mg :
-
Mass fractions of CH4 in the gas phase [−]
- ω wa :
-
Mass fractions of water in the aqueous phase [−]
- ω wg :
-
Mass fractions of water in the aqueous phase [−]
- \({\phi}\) :
-
Porosity [−]
- ρ a :
-
Density of the aqueous phase [ML−3]
- ρ c :
-
Density of CO2 [ML−3]
- ρ g :
-
Density of the gas phase [ML−3]
- c b :
-
Brine compressibility [M−1LT2]
- c m :
-
CH4 compressibility [M−1LT2]
- c r :
-
Rock compressibility [M−1LT2]
- E 1 :
-
First-order exponential integral function [−]
- f a :
-
Fractional flow of aqueous phase [−]
- f g :
-
Fractional flow of gas phase [−]
- h :
-
Thickness of the formation [L]
- k :
-
Permeability [L2]
- k ra0 :
-
End-point relative permeability for aqueous phase [−]
- k ra :
-
Relative permeabilities of the aqueous phase [−]
- k rg0 :
-
End-point relative permeability for gas phase [−]
- k rg :
-
Relative permeabilities of the gas phase [−]
- m :
-
Power-law exponents for the aqueous phase [−]
- M 0 :
-
Mass injection rate [MT−1]
- n :
-
Power-law exponents for the gas phase [−]
- P :
-
Fluid pressure [ML−1T−2]
- P 0 :
-
Initial brine aquifer pressure [ML−1T−2]
- q a :
-
Volumetric flux of the aqueous phase [LT−1]
- q D :
-
Ratio of volumetric flow rate at each region to injection flow rate [−]
- q g :
-
Volumetric flux of the gas phase [LT−1]
- r :
-
Radial distance [L]
- r E :
-
Radial extent of aquifer [L]
- S a :
-
Volumetric saturation of the aqueous phase [−]
- S g :
-
Volumetric saturation of the gas phase [−]
- S s :
-
Volumetric saturation of precipitated salt [−]
- S ar :
-
Residual aqueous phase saturation [−]
- S gc :
-
Critical gas saturation [−]
- t :
-
Time [T]
- z :
-
Similarity transform parameter [−]
References
Battistelli A., Marcolini M.: TMGAS: a new TOUGH2 EOS module for the numerical simulation of gas mixtures injection in geological structures. Int. J. Greenh. Gas Control 3, 481–493 (2009). doi:10.1016/j.ijggc.2008.12.002
Computer Modeling Group Ltd.: Calgary, Canada http://www.cmgroup.com/ (2011)
Doughty C.: Modeling geologic storage of carbon dioxide: comparison of non-hysteretic and hysteretic characteristic curves. Energy Convers Manage. 48(6), 1768–1781 (2007)
Doughty C., Freifeld B.M., Trautz R.C.: Site characterization for CO2 geologic storage and vice versa: the Frio brine pilot, Texas, USA as a case study. Environ. Geol. 54, 1635–1656 (2008)
Furati K.M.: History effect on oil recovery efficiency. J. Petroleum Sci. Eng. 19, 295–308 (1998)
Ghaderi S.M., Keith D.W., Lavoie R., Leonenko Y.: Evolution of hydrogen sulfide in sour aquifers during carbon dioxide sequestration. Int. J. Greenhouse Gas Control 5, 347–355 (2011). doi:10.1016/j.ijggc.2010.09.008
Griggs, J.: A reevaluation of geopressurized-geothermal aquifers as an energy source. In: Proceeding, workshop of geothermal reservoir engineering, Stanford University, Stanford, SGP-TR-176 (2005)
Hovorka S.D., Benson S.M., Doughty C., Freifeld B.M., Sakurai S., Daley T.M., Kharaka Y.K., Holtz M.H., Trautz R.C., Nance H.S., Myer L.R., Knauss K.G.: Measuring permanence of CO2 storage in saline formations: the Frio experiment. Environ. Geosci. 13(2), 1–17 (2006)
Littke R., Cramer B., Gerlin P., Lopatin N.V., Poelchau H.S., Schaefer R.G., Welte D.H.: Gas generation and accumulation in the West Siberian Basin. AAPG Bull. 83, 1642–1665 (1999)
Lu J., Cook P.J., Hosseini S.A., Yang C., Romanak K., Zhang T., Freifeld B.M., Smyth R., Zenga H., Hovorka S.D.: Reveal sinuous fluid flow by monitoring CO2 injection in a fluvial reservoir. J. Geophys. Res. 117, B03208 (2012). doi:10.1029/2011JB008939
Mallision B.T., Gerritsen M.G., Jessen K., Orr F.M. Jr: High order upwind schemes for two-phase, multicomponent flow. SPE J. 10(3), 297–311 (2003)
Manceau J.-C., Rohmer J.: Analytical solution incorporating history-dependent processes for quick assessment of capillary trapping during CO2 geological storage. Transp. Porous Media 90, 721–740 (2011). doi:10.1007/s11242-011-9812-z
Manrique, J.F., Kanecki, T.: Reservoir management strategies for development of gas dissolved in water (brine) reservoirs. SPE-59420-MS, doi:10.2118/59420-MS (2000)
Mathias S.A., Hardisty P.E., Trudell M.R., Zimmerman R.W.: Screening and selection of sites for CO2 sequestration based on pressure buildup. Int. J. Greenhouse Gas Control 3, 577–585 (2009a). doi:10.1016/j.ijggc.2009.05.002
Mathias S.A., Hardisty P.E., Trudell M.R., Zimmerman R.W.: Approximate solutions for pressure buildup during CO2 injection in brine aquifers. Transp. Porous Media 79, 265–284 (2009b). doi:10.1007/s11242-008-9316-7
Mathias, S.A., Gluyas, J.G., Gonzalez Martinez de Miguel, G.J., Hosseini, S.A.: Role of miscibility on pressure buildup due to constant rate injection of CO2 into closed and open brine aquifer. Water Resour. Res. 47, W12525. doi:10.1029/2011WR011051 (2011a)
Mathias S.A., Gonzalez Martinez de Miguel G.J., Tatcher K.E., Zimmerman R.W.: Pressure buidup during CO2 injection into a closed brine aquifer. Transp. Porous Med 89, 383–397 (2011b). doi:10.1007/s11242-011-9776-z
Noh, M., Lake, L.W., Bryant, S.L., Araque-Martinez, A.: Implications of coupling fractional flow and geochemistry for CO2 injection in aquifers. SPE Res. Eval. Eng. 10(4), 406–414. SPE-89341-PA (2007)
Nordbotten J.M., Celia M.A.: Similarity solutions for fluid injection into confined aquifers. J. Fluid Mech. 561, 307–327 (2006)
Oldenburg C.M., Pruess K., Benson S.M.: Process modeling of CO2 injection into natural gas reservoirs for carbon sequestration and enhanced gas recovery. Energy Fuels 15, 293–298 (2001). doi:10.1021/ef000247h
Oldenburg C.M., Doughty C.: Injection, flow and mixing of CO2 in porous media with residual gas. Trans. Porous Med 90, 201–211 (2011). doi:10.1007/s11242-010-9645-1
Orr F.M. Jr: Theory of gas injection processes. Tie-Line Publications, Copenhagen (2007)
Ramaswamy V., Boucher O., Haigh J., Hauglustaine D., Haywood J., Myhre G., Nakajima T., Shi G.Y., Solomon S.: The Scientific Basis. Contribution of Working Group I to the Third Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge University Press, Cambridge (2001)
Seto, C.J., Jessen, K., Orr, F.M.: A multicomponent, two-phase-flow model for CO2 storage and enhanced coalbed-CH4 recovery. SPE J. 14(1), 30–40, SPE-102376-PA (2009)
Seto, C.J.: Analytical theory for two-phase, multicomponent flow in porous media with adsorption, PhD dissertation, Stanford University, Stanford (2007)
Taggart, I.: Extraction of dissolved CH4 in brines by CO2 injection: implications for CO2 sequestration. SPE Res. Eval. Eng. 13(5), 791–804, SPE-124630-PA. doi:10.2118/124630-PA (2010)
Warren J.K.: Evaporates: Sediments, Resources and Hydrocarbons. Springer, Dordrecht (2006)
Xu T., Kharaka Y.K., Doughty C., Freifeld B.M., Daley T.M.: Reactive transport modeling to study changes in water chemistry induced by CO2 injection at the Frio-I brine pilot. Chem. Geol. 271(3–4), 153–164 (2010)
Zeidouni M., Pooladi-Darvish M., Keith D.: Analytical solution to evaluate salt precipitation during CO2 injection in saline aquifers. Int. J. Greenhouse Gas Control 3, 600–611 (2009). doi:10.1016/j.ijggc.2009.04.004
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hosseini, S.A., Mathias, S.A. & Javadpour, F. Analytical Model for CO2 Injection into Brine Aquifers-Containing Residual CH4 . Transp Porous Med 94, 795–815 (2012). https://doi.org/10.1007/s11242-012-0025-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11242-012-0025-x