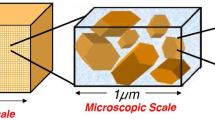
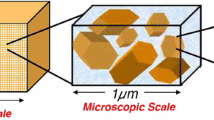
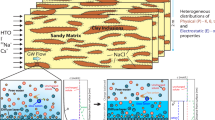
Abstract
In part I (Lima et al., Transp Porous Media, 2009), a three-scale model governing the movement of an aqueous saline solution containing four monovalent species (Na+, H+, Cl−, OH−) in kaolinite clays was derived. Unlike purely macroscopic approaches, the novelty of the formulation relied on the double averaging of the nanoscopic electro- chemistry of particle/electrolyte solution interface ruled by the electrical double layer coupled with protonation/deprotonation reactions. The passage from the nano to the micro (pore)-scale gave rise to ion-sorbed concentrations and slip velocity at the solid/fluid interface which are coupled with the microscopic Stokes problem and Nernst–Planck equations governing the hydrodynamics and ion transport in the micropores. Application of a formal homogenization procedure led to macroscopic governing equations with effective electro-chemical parameters, such as retardation coefficients, electro-osmotic permeability, and electric conductivity. In this study, we reconstruct the constitutive laws of the macroscopic coefficients by solving the nano and microscopic closure problems. New generalized isotherms for Na+ and H+ − OH− sorption are build-up based on a perturbation approach and the limitations of classical Freundlich isotherm for modeling ion sorption at the solid/fluid interface are discussed. The macroscopic governing equations are discretized by the finite volume method and numerical simulations of a transient electroosmosis experiment for desalination of a clay sample by electrokinetics are presented.
Similar content being viewed by others
Abbreviations
- a fs :
-
Surface area [m−1]
- f :
-
Characteristic function
- m, n, p:
-
Exponents of the adsorption isotherms
- k e :
-
Isoelectric point
- p :
-
Thermodynamic pressure [Pa]
- t :
-
Time [s]
- x, y:
-
Parallel and orthogonal coordinates [m]
- u :
-
Auxiliary perturbation parameter
- C ib :
-
Ionic bulk concentration with i = Na,H [mol/m3]
- \({C_{\rm H^{+}_{0}}}\) :
-
H+ concentration at the solid surface [mol/l]
- C b :
-
Bulk concentration [mol/m3]
- F :
-
Faraday constant [C/mol]
- H :
-
Half distance between the particles [m]
- K :
-
Equilibrium constant [l/mol]
- K W :
-
Ionic product of water [(mol/l)2]
- L D :
-
Debye’s length [m]
- L :
-
Sample length [m]
- M :
-
Metallic ions at the surface
- R :
-
ideal gas constant [J/(mol K)]
- R N , R H :
-
Ionic retardation coefficients
- T :
-
Temperature [K]
- Y :
-
Cell domain
- Y f , Ys:
-
Fluid and solid subdomains
- ∂Y fs :
-
Fluid/solid interface
- x :
-
Macroscopic coordinate [m]
- y :
-
Microscopic coordinate [m]
- A eff :
-
First Onsager coefficient [(C·m2)/(mol·s)]
- B eff :
-
Second Onsager coefficient [(C·m2)/(mol·s)]
- C eff :
-
Effective electric conductivity [C/(m·s)]
- \({{\bf \widehat{D}^{\rm eff}}}\) :
-
Net effective diffusivity H+–OH+ [m2/s]
- \({{\bf D^{\rm eff}_{\rm Na}}}\) :
-
Effective sodium diffusivity [m2/s]
- \({{\bf K_{P}^{\rm eff}}}\) :
-
Hydraulic conductivity [m2/(Pa·s)]
- \({{\bf K_{E}^{\rm eff}}}\) :
-
Electroosmotic permeability [m/(V·s)]
- \({{\bf I_f^{\rm eff}}}\) :
-
Effective electric current [C/(m2·s)]
- J eff :
-
Effective ionic flux [mol/(m2·s)]
- V D :
-
Darcy’s velocity [m/s]
- \({{\bf I_f^{\rm eff}}}\) :
-
Spatial gradients
- Δx :
-
Mesh size
- Δt :
-
Time step
- \({< \cdot >}\) :
-
Volumetric average operator
- \({< \cdot >_{\rm fs}}\) :
-
Interfacial average operator
- Na+, H+, Cl−, OH− :
-
Ionic species
- α, β:
-
Electro-chemical coefficients
- δ :
-
Half particle thickness [m]
- \({\epsilon}\) :
-
Perturbation parameter
- \({\tilde{\epsilon}_{0}}\) :
-
Permittivity of the free space [C/(V m)]
- \({\tilde{\epsilon}_{r}}\) :
-
Dielectric constant
- \({\phi}\) :
-
Macroscopic electric potential [V]
- \({\overline{\phi}}\) :
-
Dimensionless macroscopic electric potential
- \({\gamma_{\rm H^{+}}}\) :
-
Protonic concentration at the surface [mol/m2]
- η :
-
Porosity
- κ P :
-
Characteristic tensorial function
- μ f :
-
Viscosity of the water [Pa·s]
- π :
-
Microscopic pressure
- σ :
-
Surface charge density [C/m2]
- ζ :
-
Zeta potential [V]
- \({{\overline \zeta}}\) :
-
Dimensionless zeta potential
- \({\Theta}\) :
-
Nonlinear parameter in the advection term of the protons
- Γ:
-
Sorbed ionic concentration at the surface [mol/m2]
- ΓMAX :
-
Density of available sites [sites/nm2]
- Ω:
-
Macroscopic domain
References
Alshawabkeh A.N., Acar Y.B.: Electrokinetic remediation: theoretical model. J. Geotech. Eng. 122, 186–196 (1996)
Auriault J.L., Lewandowska J.: Diffusion/adsorption/advection macrotransport in soils. Eur. J. Mech. A 15, 681–704 (1996)
Avena M.J., De Pauli C.P.: Modeling the interfacial properties of an amorphous aluminosilicate dispersed in aqueous NaCl solution. Colloids Surf. A. 118, 75–87 (1996)
Beddiar K., Fen-Chong T., Dupas A., Berthaud Y., Dangla P.: Role of pH in electro-osmosis: experimental study on NaCl–water saturated kaolinite. Transp. Porous Media 61(1), 93–107 (2005)
Dangla P., Chong T.F., Gaulard F.: Modelling of pH-dependent electro-osmotic flows. C. R. Mecanique 332, 915–920 (2004)
Dormieux L., Barboux P., Coussy O., Dangla P.: A macroscopic model of the swelling phenomenon of a saturated clay. Eur. J. Mech. A 14(6), 981–1004 (1995)
Edwards D.A.: Charge transport through a spatially periodic porous medium: electrokinetic and convective dispersion phenomena. Phil. Trans. R. Soc. Lond. A. 353, 174–180 (1995)
Finno R.J., Chung K., Yin J., Feldkamp J.R.: Coefficient of permeability from AC electroosmosis experiments: 2 Results. J. Geotech. Eng. ASCE 122(5), 355–364 (1996)
Ganor J., Cama J., Metz V.: Surface Protonation data of Kaolinite: reevaluation based on dissolution experiments. J. Colloid Interface Sci. 264, 67–75 (2003)
Goldberg S., Criscenti L.J., Turner D.R., Davis J.A., Cantrell K.J.: Adsorptioná1-desorption processes in subsurface reactive transport modeling. Vadose Zone J 6, 407–435 (2007)
Hlushkou D., Morgenstern A.S., Tallarek U.: Numerical analysis of electroosmotic flow in dense regular and random arrays of impermeable nonconducting spheres. Langmuir 21, 6097–6112 (2005)
Huertas F.J., Chou L., Wollast R.: Mechanism of kaolinite dissolution at room temperature an in pressure: part 1. Surface speciation. Geochim. Cosmochim. Acta. 62, 417–431 (1997)
Lemaire T., Moyne C., Stemmelen D.: Modelling of electro-osmosis in clayey materials including pH effects. Phys. Chem. Earth 32, 441–452 (2007)
Lide D.R.: Handbook of Chemistry and Physics. CRC Press, Florida (2006)
Lima S.A., Murad M.A., Moyne C., Stemmelen D.: A three scale model of pH-dependent steady flows in 1:1 clays. Acta Geotech. 3, 153–174 (2008)
Lima, S.A., Murad, M. A., Moyne, C., Stemmelen, D.: A three scale model of pH-dependent flow and ion transport with equilibrium adsorption in Kaolinite clays: I Homogenization analysis. Transp. Porous Media (2009). doi:10.1007/s11242-010-9545-4
Ma C., Eggleton R.A.: Cation exchange capacity of kaolinite. Clays Clay Miner. 47(2), 174–180 (1999)
Mitchell J.: Fundamentals of Soil Behavior. Wiley, New York (1993)
Moyne C., Murad M.A.: A two-scale model for coupled electro-chemo-mechanical phenomena and Onsager’s reciprocity relations in expansive clays: I homogenization analysis. Transp. Porous Media 62(3), 333–380 (2006a)
Moyne C., Murad M.A.: A two-scale model for coupled electro-chemo-mechanical phenomena and onsager’s reciprocity relations in expansive clays: II computational validation. Transp. Porous Media 63(1), 13–56 (2006b)
Murad M.A., Moyne C.: A dual-porosity model for ionic solute transport in expansive clays. Comput. Geosci. 12, 47–82 (2008)
Patankar S.V.: Numerical Heat Transfer and Fluid Flow. Hemisphere Publishing Corporation, Washington, DC (1980)
Samson E., Marchand J., Robert J-L., Bournazel J.-P.: Modelling Ion Diffusion Mechanisms in Porous Media. Int. J. Numer. Methods Eng. 46, 2043–2060 (1999)
Sanchez-Palencia E.: Non-Homogeneous Media and Vibration Theory. Lectures Notes in Physics. Springer, Berlin (1980)
Yeung A.T., Mitchell J.K.: Coupled fluid, electrical and chemical flows in soil. Geotechnique 43(1), 121–134 (1993)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
de Lima, S.A., Murad, M.A., Moyne, C. et al. A Three-Scale Model of pH-Dependent Flows and Ion Transport with Equilibrium Adsorption in Kaolinite Clays: II Effective-Medium Behavior. Transp Porous Med 85, 45–78 (2010). https://doi.org/10.1007/s11242-010-9546-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11242-010-9546-3