Abstract
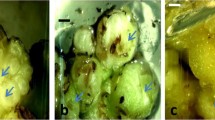
The development of an efficient and consistent callus-mediated in vitro regeneration protocol is crucial for biotechnological approaches aimed at improving chickpea, an economically important crop legume. In this study, we assess the effectiveness of callus-mediated regeneration in different chickpea genotypes. Through in vitro screening of explants, we identified the Indian cultivar Pusa 240 as a favourable genotype with higher efficiency of somatic embryogenesis and in vitro plant regeneration. Building upon this finding, we have successfully established two distinct protocols for chickpea callus-mediated somatic embryogenesis, utilizing leaf and hypocotyl explants obtained from the Pusa 240 genotype. These protocols achieved plant regeneration efficiencies of 27% using leaf explants and 46.6 − 66% using hypocotyl explants. Extensive literature review and comparative analysis underscored the superiority of our current protocol. Subsequently, the regenerated plants were successfully acclimatized and transferred to the greenhouse, exhibiting normal phenotypic growth. This detailed regeneration method will provide a valuable resource for chickpea genetic transformations and the generation of large mutant populations where embryogenesis via callus formation is required. The protocol presented here establishes a powerful tool for studying the functional genomics of chickpea plants and lays the foundation for future advancements in this field.
Key message
Identification of the efficient genotype and development of an efficient callus-mediated somatic embryogenesis protocol for chickpea transformation. An essential milestone towards chickpea Tnt1 based mutant population generation.




Similar content being viewed by others
Data availability
This manuscript has data included as electronic supplemental file.
Abbreviations
- 2,4-D:
-
2,4-Dichlorophenoxyacetic acid
- BAP:
-
6-benzylaminopurine
- CIM:
-
Callus-inducing medium
- CK-like:
-
Cytokinin-like
- GA3 :
-
Gibberellic acid 3
- IBA:
-
Indole-3-butyric acid
- MS:
-
Murashige and Skoog (1962) medium
- NAA:
-
a-naphthalene acetic acid
- RIM:
-
Root-inducing medium
- SH:
-
Schenk-Hildebrandt (1972) medium
- SIM:
-
Shoot-inducing medium
- SEM:
-
Shoot elongation medium
- TDZ:
-
Thidiazuron
References
Amani MR, Zebarjadi A et al (2022) Somatic embryogenesis and β-glucuronidase transformation in chickpea (Cicer arietinum Cv. Bivanich). Mol Bio Rep 49(12):11219–11227
Arora A, Chawla HS (2005) Organogenic plant regeneration via callus induction in chickpea (Cicer arietinum L.)—Role of genotypes, growth regulators and explants. Ind J Biotech 4:251–256
Arriagada O, Cacciuttolo F et al (2022) A Comprehensive Review on Chickpea (Cicer arietinum L.) breeding for abiotic stress tolerance and climate change resilience. Int J Mol Sci 23(12):6794
Atif RM, Patat-Ochatt EM et al (2012) Gene transfer in legumesProgress in Botany, vol 74. Springer, pp 37–100
Barceló M, El-Mansouri I et al (1998) Regeneration and transformation via Agrobacterium tumefaciens of the strawberry cultivar Chandler. Plant Cell Tiss Organ Cult 54:29–36
Barna KS, Wakhlu AK (1994) Whole plant regeneration of Cicer arietinum from callus cultures via organogenesis. Plant Cell Rep 13:510–513
Bhowmik P, Konkin D et al (2021) CRISPR/Cas9 gene editing in legume crops: opportunities and challenges. Legume Sci 3(3):e96
Chakraborti D, Sarkar A et al (2006) Efficient and rapid in vitro plant regeneration system for Indian cultivars of chickpea (Cicer arietinum L). Plant Cell Tiss Organ Cult 86:117–123
Chenar HM, Kahrizi D et al (2016) Callus induction is affected by explant type and plant growth regulators in chickpea (Cicer arietinum L). Bih Biologist 10(1):28–32
Cosson V, Eschstruth A et al (2015) Medicago truncatula transformation using leaf explants. Agrobacterium Protocols: Volume 1:43–56
Courtial B, Feuerbach F et al (2001) Tnt1 transposition events are induced by in vitro transformation of Arabidopsis thaliana, and transposed copies integrate into genes. Mol Genet Genom 265:32–42
Cui Y, Barampuram S et al (2013) Tnt1 retrotransposon mutagenesis: a tool for soybean functional genomics. Plant Physiol 161(1):36–47
d’Erfurth I, Cosson V et al (2003) Efficient transposition of the Tnt1 Tobacco retrotransposon in the model legume Medicago truncatula. Plant J 34(1):95–106
Das Bhowmik SS, Cheng AY et al (2019) Robust genetic transformation system to obtain non-chimeric transgenic chickpea. Front Plant Sci 10:524
Détain A, Bhowmik P et al (2022) Latest biotechnology tools and targets for improving abiotic stress tolerance in protein legumes. Environ Exp Bot 197:104824
Duangpan S, Zhang W et al (2013) Insertional mutagenesis using Tnt1 retrotransposon in potato. Plant Physiol 163(1):21–29
FAOSTAT (2019) Statistical database of the United Nations. Food and Agricultural Organization (FAO)
Fras A, Smolen B et al (2008) Vascularization of zygotic and somatic embryos. Acta Biologica Cracoviensia Series Botanica 50:43–48
GOI (2021) Agricultural statistics at a glance 2020, Ministry of Agriculture & Farmers Welfare. Department of Agriculture & Farmers Welfare, Directorate of Economics & Statistics
Graham PH, Vance CP (2003) Legumes: importance and constraints to greater use. Plant Physiol 131(3):872–877
Gupta SD, Agarwal A (2017) Influence of LED lighting on in vitro plant regeneration and associated cellular redox balance. Light emitting diodes for agriculture: smart lighting: 273–303
Gupta SD, Jatothu B (2013) Fundamentals and applications of light-emitting diodes (LEDs) in in vitro plant growth and morphogenesis. Plant Biotechnol Rep 7(3):211–220
Hoffmann B, Trinh TH et al (1997) A new Medicago truncatula line with superior in vitro regeneration, transformation, and symbiotic properties isolated through cell culture selection. Mol Plant-Microbe Inter 10(3):307–315
Iantcheva A, Revalska M et al (2016) Tnt1 retrotransposon as an efficient tool for development of an insertional mutant collection of Lotus japonicus. In Vitro Cell Develop Biol-Plant 52:338–347
Jha UC, Nayyar H et al (2022) Progress of genomics-driven approaches for sustaining underutilized legume crops in the post-genomic era. Front Genet 13:536
Khandal H, Gupta SK et al (2020) Root-specific expression of chickpea cytokinin oxidase/dehydrogenase 6 leads to enhanced root growth, drought tolerance and yield without compromising nodulation. Plant Biotech J 18(11):2225–2240
Koul B, Sharma K et al (2022) Chickpea (Cicer arietinum L.) Biology and Biotechnology: from domestication to Biofortification and Biopharming. Plants 11(21):2926
Landi L, Mezzetti B (2006) TDZ, auxin and genotype effects on leaf organogenesis in Fragaria. Plant Cell Rep 25:281–288
Lazzeri PA, Hildebrand DF et al (1988) Soybean somatic embryogenesis: interactions between sucrose and auxin. Plant Cell Rep 7:517–520
Lee YH, Kim HS et al (2004) A new selection method for pepper transformation: callus-mediated shoot formation. Plant Cell Rep 23:50–58
Mahto RK, Singh C et al (2022) Chickpea biofortification for cytokinin dehydrogenase via genome editing to enhance abiotic-biotic stress tolerance and food security. Front Genet 13
Martinez ME, Jorquera L et al (2021) Effect of the carbon source and plant growth regulators (PGRs) in the induction and maintenance of an in vitro callus culture of Taraxacum officinale (L) Weber Ex FH Wigg. Agronomy 11(6):1181
Mazier M, Botton E et al (2007) Successful gene tagging in lettuce using the Tnt1 retrotransposon from Tobacco. Plant Physiol 144(1):18–31
Murthy BNS, Murch SJ et al (1998) Thidiazuron: a potent regulator of in vitro plant morphogenesis. In Vitro Cell Develop Biol-Plant 34:267–275
Nandety RS, Serrani-Yarce JC et al (2020) Insertional mutagenesis of Brachypodium distachyon using the Tnt1 retrotransposable element. Plant J 103(5):1924–1936
O’Malley RC, Ecker JR (2010) Linking genotype to phenotype using the Arabidopsis unimutant collection. Plant J 61(6):928–940
Ochatt S, Conreux C et al (2018) Phytosulfokine-alpha, an enhancer of in vitro regeneration competence in recalcitrant legumes. Plant Cell Tiss Organ Cult 135:189–201
Oosumi T, Gruszewski HA et al (2006) High-efficiency transformation of the diploid strawberry (Fragaria vesca) for functional genomics. Planta 223:1219–1230
Patra AP, Samal KC et al (2023) Agrobacterium tumefaciens-mediated Genetic Transformation of Green Gram [Vigna radiata (L.) Wilczek]-A recalcitrant grain legume. Legume Res-Inter J 1:7
Peoples MB, Brockwell J et al (2009) The contributions of nitrogen-fixing crop legumes to the productivity of agricultural systems. Symbiosis 48:1–17
Pouteau S, Spielmann A et al (1991) Effects of Tnt1 Tobacco retrotransposon insertion on target gene transcription. Mol Gen Genet 228:233–239
Report A (2022) -23) Department of Agriculture & Farmers Welfare, Ministry of Agriculture & Farmers Welfare, Government of India
Reuveni M, Evenor D (2007) On the effect of light on shoot regeneration in petunia. Plant Cell Tiss Organ Cult 89:49–54
Roorkiwal M, Von Wettberg EJ et al (2014) Exploring germplasm diversity to understand the domestication process in Cicer spp. using SNP and DArT markers. PLoS ONE 9(7):e102016
Sagare AP, Suhasini K et al (1993) Plant regeneration via somatic embryogenesis in chick pea (Cicer arietinum L). Plant Cell Rep 12:652–655
Schenk RU, Hildebrandt AC (1972) Medium and techniques for induction and growth of monocotyledonous and dicotyledonous plant cell cultures. Can J Bot 50(1):199–204
Somers DA, Samac DA et al (2003) Recent advances in legume transformation. Plant Physiol 131(3):892–899
Suhasini K, Sagare AP et al (1997) Comparative study of the development of zygotic and somatic embryos of chickpea (Cicer arietinum L). Plant Sci 128(2):207–216
Sun L, Gill US et al (2019) Genome-wide analysis of flanking sequences reveals that Tnt1 insertion is positively correlated with gene methylation in Medicago truncatula. Plant J 98(6):1106–1119
Svetleva D, Velcheva M et al (2003) Biotechnology as a useful tool in common bean (Phaseolus vulgaris L.) improvement. Euphytica 131:189–200
Tadege M, Wen J et al (2008) Large-scale insertional mutagenesis using the Tnt1 retrotransposon in the model legume Medicago truncatula. Plant J 54(2):335–347
Tripathi L, Singh AK et al (2013) Optimization of regeneration and Agrobacterium-mediated transformation of immature cotyledons of chickpea (Cicer arietinum L). Plant Cell Tiss Organ Cult 113:513–527
Urbański DF, Małolepszy A et al (2012) Genome-wide LORE1 retrotransposon mutagenesis and high‐throughput insertion detection in Lotus japonicus. Plant J 69(4):731–741
Varshney RK, Song C et al (2013) Draft genome sequence of chickpea (Cicer arietinum) provides a resource for trait improvement. Nat Biotech 31(3):240–246
Vashishat RK, Chowdhury JB et al (1998) Multiple shoots from cotyledonary node explants of non-nodulating genotype (ICC435M) of chickpea, Cicer arietinum L. Ind J Exp Biol 36(12):1276–1279
Vives C, Charlot F et al (2016) Highly efficient gene tagging in the bryophyte Physcomitrella patens using the Tobacco (Nicotiana tabacum) Tnt1 retrotransposon. New Phytol 212(3):759–769
Wardhani TAK, Roswanjaya YP et al (2019) Transforming, genome editing and phenotyping the nitrogen-fixing tropical Cannabaceae tree Parasponia andersonii. J Vis Exp (150)
Zhang Q, Hui DU et al (2018) Efficient transposition of the Retrotransposon Tnt1 in Cucumber (Cucumis sativus L). Hort Plant J 4(3):111–116
Acknowledgements
The authors would like to thank Dr S.K. Parida, NIPGR New Delhi, for providing chickpea seeds. We acknowledge NIPGR Phytotron Facility; CIF-NIPGR and DBT (Department of Biotechnology)-eLibrary Consortium (DeLCON), India, for providing access to e-resources. We acknowledge the technical assistance of Pradeep Maurya.
Funding
This work was financially supported by research grants from NIPGR core and DBT (BT/PR38393/GET/119/302/2020).
Author information
Authors and Affiliations
Contributions
The conception of the study and experiments were designed by SS, MSK and DC. VKJ performed experiments, analysed data, and prepared the figures. VKJ and SS wrote the manuscript. All authors commented on and contributed to the revision of the manuscript. All authors read and approved the manuscript for publication.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest.
Compliance with ethical standards
The work was conducted as per the Institutional Biosafety Committee (IBSC) protocol.
Additional information
Communicated by Sergio J. Ochatt.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.



Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Jangid, V.K., Senthil-Kumar, M., Chandran, D. et al. Callus induction and efficient in vitro plant regeneration protocol for Chickpea. Plant Cell Tiss Organ Cult 156, 21 (2024). https://doi.org/10.1007/s11240-023-02633-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11240-023-02633-0