Abstract
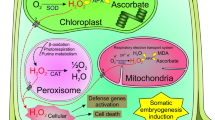
A major barrier to plantlet regeneration using somatic embryogenesis technology in Pinus massoniana is the recalcitrance to achieve efficient proliferation and germination of embryogenic cells, resulting from the loss of embryogenic potential (capability to form viable somatic embryos) during successive proliferation culture. Here, an integrative analysis of molecular and physiological mechanisms on the loss of embryogenic potential following long-term proliferation culture was performed to identify key factors involved in influencing proliferation and germination of embryogenic cells. Embryogenic potential of cells, which was identified by proliferation coefficient (PC) and germination percentage (GP) at the present study, was distinct between the 10th (S10, PC: 11.0, GP: 66.4%) and 30th (S30, PC: 1.3, GP: 4.6%) proliferation cycle given the duration of proliferation cycle was 14 days. Transcriptome analysis indicated 99.6% differentially expressed genes in reactive oxygen species production pathway were upregulated. Following an application of 1.0 mM antioxidant against H2O2, L-glutathione, in the culture media, increases in both PC and GP were investigated for S30 embryogenic cells. Further evidence revealed that H2O2 regulated Ca2+ signal by increasing Ca2+ influx and decreasing Ca2+ efflux, resulting in cytosolic Ca2+ accumulation. With the reduction of Ca2+ concentration in media, both PC and GP of embryogenic cells were significantly enhanced. Our findings suggest that H2O2 and Ca2+ get involved in the loss of embryogenic potential during long-term proliferation culture. We have developed information demonstrating that regulation of Ca2+ level and oxidation environment in vitro can enhance proliferation and germination of embryogenic cells in P. massoniana.
Key message
H2O2 and Ca2+ got involved in the embryogenic potential loss of cells during successive proliferation culture in Pinus massoniana by comparing transcriptomes, H2O2 and Ca2+ flux, and Ca2+ subcellular localization.












Similar content being viewed by others
Data Availability
RNA-seq datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: NCBI Sequence Read Archive (SRA) platform (http://trace.ncbi.nlm.nih.gov/Traces/sra/) under the accession number PRJNA761875. In addition, other original contributions presented in the study are available from the corresponding author upon request.
References
Belmonte MF, Macey J, Yeung EC, Stasolla C (2005) The effect of osmoticum on ascorbate and glutathione metabolism during white spruce (Picea glauca) somatic embryo development. Plant Physiol Biochem 43:337–346
Belmonte MF, Ambrose SJ, Ross ARS, Abrams SR, Stasolla C (2006) Improved development of microspore-derived embryo cultures of Brassica napus cv Topaz following changes in glutathione metabolism. Physiol Plant 127:690–700
Carpenter CV, Koester MK, Blackman S, Gupta PK (2000) Method of determining conifer embryo maturity using sugar alcohol content. US Patent 6,150,167, issued 21 November 2000
Chen YY, Cao CJ, Guo ZJ et al (2020) Herbivore exposure alters ion fluxes and improves salt tolerance in a desert shrub. Plant Cell Environ 43:400–419
Cnubben NP, Rietjens IM, Wortelboer H (2001) The interplay of glutathione-related processes in antioxidant defense. Environ Toxicol Phar 10:141–152
Dan Y (2008) Biological functions of antioxidants in plant transformation. In Vitro Cell Dev Bio Plant 44:149–161
De Gara L, De Pinto MC, Moliterni VMC, D’Egidio MG (2003) Redox regulation and storage processes during maturation in kernels of Triticum durum. J Exp Bot 54:249–258
Demidchik V, Shabala SN, Davies JM (2007) Spatial variation in H2O2 response of Arabidopsis thaliana root epidermal Ca2+ flux and plasma membrane Ca2+ channels. Plant J 49:377–386
Gupta PK, Durzan DJ (1985) Shoot multiplication from mature trees of Douglas-fir (Pseudotsuga menziesii) and sugar pine (Pinus lambertiana). Plant Cell Rep 4:177–179
He GW, Li WG, Wang RX, Zhang CG (2014) Development and application of triphenyltetrazolium chloride (TTC) method for determining the survival of cultured cells. Mil Med Sci 2014 5:388–391
Hübner C, Haase H (2021) Interactions of zinc- and redox-signaling pathways. Redox Biol. https://doi.org/10.1016/j.redox.2021.101916
Kamata H, Hirata H (1999) Redox regulation of cellular signaling. Cell Signal 1:1–14
Kissoudis C, Chowdhury R, van Heusden S et al (2015) Combined biotic and abiotic stress resistance in tomato. Euphytica 2:317–332
Li DM, Wang YQ, Ye XL, Liang CY (2010) Ultracytochemical localization of calcium during embryo sac development in Phaius tankervilliae (Orchidaceae). Acta Bot Yunnanica 6:495–502
Ma J, Wei L, Li J, Li H (2018) The analysis of genes and phytohormone metabolic pathways associated with leaf shape development in Liriodendron chinense via De novo transcriptome sequencing. Genes 9:577. https://doi.org/10.3390/genes9120577
Mammucari C (2021) In the right place at the right time: ROS and ca are allies in the battle for survival. Cell Calcium. https://doi.org/10.1016/j.ceca.2021.102354
McLamore ES, Porterfield DM (2011) Non-invasive tools for measuring metabolism and biophysical analyte transport: selfreferencing physiological sensing. Chem Soc Rev 40:5308–5320
Meiter A (1998) Glutathione metabolism and its selective modification. J Biol Chem 26:17205–17206
Mori IC, Schroeder JI (2004) Reactive oxygen species activation of plant Ca2+ channels: a signaling mechanism in polar growth, hormone transduction, stress signaling, and hypothetically mechanotransduction. Plant Physiol 135:702–708
Ni ZX, Han X, Yang ZQ et al (2020) Integrative analysis of wood biomass and developing xylem transcriptome provide insights into mechanisms of lignin biosynthesis in wood formation of Pinus massoniana. Int J Biol Macromol 163:1926–1937
Pullman GS, Buchanan M (2003) Loblolly pine (Pinus taeda L.): stagespecific elemental analyses of zygotic embryos and female gametophyte tissue. Plant Sci 164:943–954
Pullman GS, Johnson S (2009a) Loblolly pine (Pinus taeda L.) female gametophyte and embryo pH changes during embryo and seed development. Tree Physiol 29:829–836
Pullman GS, Johnson S (2009b) Osmotic measurements in whole megagametophytes and embryos of loblolly pine (Pinus taeda L.) during embryo and seed development. Tree Physiol 29:819–827
Pullman GS, Zeng X, Copeland-Kamp B et al (2015) Conifer somatic embryogenesis: improvements by supplementation of medium with oxidation-reduction agents. Tree Physiol 35:209–224
Shabala S, Wu H, Bose J (2015) Salt stress sensing and early signaling events in plant roots: current knowledge and hypothesis. Plant Sci 241:109–119
Shoresh M, Spivak M, Bernstein N (2011) Involvement of calcium-mediated effects on ROS metabolism in the regulation of growth improvement under salinity. Free Radical Bio Med 51:1221–1234
Stasolla C (2010) Changes in the glutathione and ascorbate redox state trigger growth during embryo development and meristem reactivation at germination. In: Anjum NA, Umar S, Chan M (eds) Ascorbate-glutathione pathways and stress tolerance in plants, XVII, pp 231–249, ISBN 978-90-481-9403-2
Sun J, Chen S, Da S et al (2009a) NaCl induced alternations of cellular and tissue ion fluxes in roots of saltresistant and salt-sensitive poplar species. Plant Physiol 2:1141–1153
Sun J, Dai S, Wang R et al (2009b) Calcium mediates root K+/Na+ homeostasis in poplar species differing in salt tolerance. Tree Physiol 9:1175–1186
Vales T, Fang X, Ge L et al (2007) Improved somatic embryo maturation in loblolly pine by monitoring ABA-responsive gene expression. Plant Cell Rep 26:133–143
Yan S, McLamore ES, Dong S et al (2015) The role of plasma membrane H+-ATPase in jasmonate-induced ion fluxes and stomatal closure in Arabidopsis thaliana. Plant J 4:638–649
Yang MH, Zhang DL, Li ZH, Jin XC, Ding GJ (2011) Somatic embryogenesis with immature embryos of masson pine (Pinus massoniana Lamb). Plant Physiol J 47:904–912
Yao RL, Wang Y (2016) An effective protocol for regenerating mature Pinus massoniana L. trees by tissue culture. Res J Biotechnol 11:75–80
Yao RL, Wang Y (2020) An advanced protocol for the establishment of plantlets originating from somatic embryos in Pinus massoniana. 3 Biotech 10:394. https://doi.org/10.1007/s13205-020-02385-0
Yuan Y, Yu M, Jia Z, Song X, Liang Y, Zhang J (2018) Analysis of Dendrobium huoshanense transcriptome unveils putative genes associated with active ingredients synthesis. BMC Genomics 19:978. https://doi.org/10.1186/s12864-018-5305-6
Zhou T, Yang X, Guo K et al (2016) ROS homeostasis regulates somatic embryogenesis via the regulation of auxin signaling in cotton. Mol Cell Proteomics 6:2108–2124
Acknowledgements
We thank the local Paiyangshan Forest Farm for their valuable fieldwork and collection of plant materials. This work was supported by the National Natural Science Foundation of China [32260381 and 31960311], Guangxi Science and Technology Project [2023GXNSFAA026449], and Guangxi Forestry Science and Technology Project [(2021)3].
Funding
This work was funded by the National Natural Science Foundation of China [32260381 and 31960311], Guangxi Science and Technology Project [2023GXNSFAA026449], and Guangxi Forestry Science and Technology Project [(2021)3].
Author information
Authors and Affiliations
Contributions
YW performed the data analysis and manuscript drafting. RY conceived the project, designed the research, and interpret the results. All authors have read and approved the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest. This article does not contain any studies with human participants or animals performed by any of the authors. Informed consent was obtained from all individual participants included in the study.
Ethical Statement
I certify that this manuscript is original and has not been published and will not be submitted elsewhere for publication while being considered by Plant Cell, Tissue and Organ Culture. And the study is not split up into several parts to increase the quantity of submissions and submitted to various journals or to one journal over time. No data have been fabricated or manipulated (including images) to support our conclusions. No data, text, or theories from others are presented as if they were our own. The submission has been received explicitly from all co-authors. And authors whose names appear on the submission have contributed sufficiently to the scientific work and therefore share collective responsibility and accountability for the results.
Additional information
Communicated by Paloma Moncaleán.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, Y., Yao, R. H2O2 and Ca2+ are involved in the embryogenic potential loss of cells during long-term proliferation culture in Pinus massoniana. Plant Cell Tiss Organ Cult 154, 657–672 (2023). https://doi.org/10.1007/s11240-023-02540-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11240-023-02540-4