Abstract
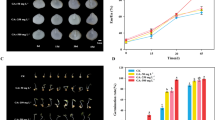
Ginsenosides Rg1 and Re are two major protopanaxatriol-type ginsenosides in Panax ginseng. Dammarendiol synthase (DDS) and protopanaxatriol synthase (PPTS) are the two key enzymes in the biosynthesis of PPT-type ginsenosides. Abscisic acid (ABA) is an important endogenous phytohormone, which is involved in abiotic stress. P. ginseng adventitious roots (PGARs) are attractive resources to be used in stress studies. However, little is known about the role of ABA on ginsenoside biosynthesis in PGARs under cold stress. In the present study, compared to control conditions (25 °C), cold stress (5 °C) induced the accumulation of ginsenosides Rg1 and Re, which was confirmed by increased expression levels of DDS and PPTS genes. Furthermore, cold stress triggered the accumulation of ABA prior to accumulation of ginsenosides Rg1 and Re. The inhibition of ABA biosynthesis with nordihydroguaiaretic acid (NDGA) in cold-exposed PGARs down-regulated expression levels of DDS and PPTS genes, and suppressed the accumulation of ginsenosides Rg1 and Re; whereas exogenous addition of ABA to NDGA-treated PGARs restored expression levels of DDS and PPTS genes, and accumulation of ginsenosides Rg1 and Re, respectively. These results indicated that ABA participation in the synthesis of ginsenosides Rg1 and Re under cold stress in PGARs. In addition, ABA treatment efficiently triggered accumulation of ginsenosides Rg1 and Re in PGARs in concentration-dependent manner at 25 °C, reaching a maximum value at 100 uM ABA, which was 2.0-fold higher than that of the control. Therefore, ABA can be used as an elicitor for the production of ginsenosides Rg1 and Re in PGARs.
Key message
Abscisic acid (ABA) was involved in cold-induced accumulation of ginsenosides Rg1 and Re in P. ginseng adventitious roots (PGARs), and exogenous application of ABA efficiently promoted accumulation of ginsenosides Rg1 and Re in PGARs.




Similar content being viewed by others
Abbreviations
- ABA:
-
Abscisic acid
- DDS:
-
Dammarendiol synthase
- DW:
-
Dry weight
- IBA:
-
Indole-3-butyric acid
- MS:
-
Murashige & Skoog
- NDGA:
-
Nordihydroguaiaretic acid
- PGARs:
-
Panax ginseng adventitious roots
- PPD:
-
Protopanaxadiol
- PPT:
-
Protopanaxatriol
- PPTS:
-
Protopanaxatriol synthase
- ROS:
-
Reactive oxygen species
References
Ali MB, Yu KW, Hahn EJ, Paek KY (2005) Differential responses of anti-oxidants enzymes, lipoxygenase activity, ascorbate content and the production of saponins in tissue cultured root of mountain Panax ginseng C.A. Mayer and Panax quinquefolium L. in bioreactor subjected to methyl jasmonate stress. Plant Sci 169:83–92
Ali MB, Hahn EJ, Paek KY (2006a) Copper-induced changes in the growth, oxidative metabolism, and saponin production in suspension culture roots of Panax ginseng in bioreactors. Plant Cell Rep 25:1122–1132
Ali MB, Yu KW, Hahn EJ, Paek KY (2006b) Methyl jasmonate and salicylic acid elicitation induces ginsenosides accumulation, enzymatic and non-enzymatic antioxidant in suspension culture Panax ginseng roots in bioreactors. Plant Cell Rep 25:613–620
Ali MB, Dewir YH, Hahn EJ, Paek KY (2008) Effect of carbon dioxide on antioxidant enzymes and ginsenoside production in root suspension cultures of Panax ginseng. Environ Exp Bot 63:297–304
Augustin JM, Kuzina V, Andersen SB, Bak S (2011) Molecular activities, biosynthesis and evolution of triterpenoid saponins. Phytochemistry 72:435–457
Baron KN, Schroeder DF, Stasolla C (2012) Transcriptional response of abscisic acid (ABA) metabolism and transport to cold and heat stress applied at the reproductive stage of development in Arabidopsis thaliana. Plant Sci 188–189:48–59
Chen XC, Zhu YG, Zhu LA, Huang C, Chen Y, Chen LM, Fang F, Zhou YC, Zhao CH (2003) Ginsenoside Rg1 attenuates dopamine-induced apoptosis in PC12 cells by suppressing oxidative stress. Eur J Pharmacol 473:1–7
Chen F, Luo J, Kong L (2012) Fast isolation of ginsenosides Re and Rg1 from the roots of Panax ginseng by HSCCC-ELSD combined with MCI gel CC guided by HPLC-MS. J Liq Chromatogr Related Technol 35:912–923
Chinese Pharmacopoeia Commission (2020) Panax ginseng. Pharmacopoeia of the People’s Republic of China, part I (Chinese). China Medical Science Press, Beijing, pp 8–9
Devi BR, Kim YJ, Selvi SK, Gayathri S, Altanzul K, Parvin S, Yang DU, Lee OR, Lee S, Yang DC (2012) Influence of potassium nitrate on antioxidant level and secondary metabolite genes under cold stress in Panax ginseng. Russ J Plant Physiol 59:318–325
Eremina M, Rozhon W, Poppenberger B (2016) Hormonal control of cold stress responses in plants. Cell Mol Life Sci 73:797–810
González-Villagra J, Kurepin LV, Reyes-Díaz MM (2017) Evaluating the involvement and interaction of abscisic acid and miRNA156 in the induction of anthocyanin biosynthesis in drought-stressed plants. Planta 246:299–312
Grant OM, Brennan DP, Mellisho Salas CD, Dix PJ (2014) Impact of enhanced capacity to scavenge reactive oxygen species on cold tolerance of tobacco. Int J Plant Sci 175:544–554
Han JY, Kwon YS, Yang DC, Jung YR, Choi YE (2006) Expression and RNA interference-induced silencing of the dammarenediol synthase gene in Panax ginseng. Plant Cell Physiol 47:1653–1662
Han JY, Kim HJ, Kwon YS, Choi YE (2011) The Cyt P450 enzyme CYP716A47 catalyzes the formation of protopanaxadiol from dammarenediol-II during ginsenoside biosynthesis in Panax ginseng. Plant Cell Physiol 52:2062–2073
Han JY, Hwang HS, Choi SW, Kim HJ, Choi YE (2012) Cytochrome P450 CYP716A53v2 catalyzes the formation of protopanaxatriol from protopanaxadiol during ginsenoside biosynthesis in Panax ginseng. Plant Cell Physiol 53:1535–1545
Hou M, Wang R, Zhao S, Wang Z (2021) Ginsenosides in Panax genus and their biosynthesis. Acta Pharm Sin B 11:1813–1834
Huang C, Qian ZG, Zhong JJ (2013) Enhancement of ginsenoside biosynthesis in cell cultures of Panax ginseng by N, N’-dicyclohexylcarbodiimide elicitation. J Biotechnol 165:30–36
Huang X, Chen MH, Yang LT, Li YR, Wu JM (2015) Effects of exogenous abscisic acid on cell membrane and endogenous hormone contents in leaves of sugarcane seedlings under cold stress. Sugar Tech 17:59–64
Huang X, Shi H, Hu Z, Liu A, Amombo E, Chen L, Fu J (2017) ABA is involved in regulation of cold stress response in Bermudagrass. Front Plant Sci. https://doi.org/10.3389/fpls.2017.01613
Ibrahim MH, Jaafar HZ (2013) Abscisic acid induced changes in production of primary and secondary metabolites, photosynthetic capacity, antioxidant capability, antioxidant enzymes and lipoxygenase inhibitory activity of Orthosiphon stamineus Benth. Molecules 18:7956–7976
Jiang M, Liu J, Quan X, Quan L, Wu S (2016) Different chilling stresses stimulated the accumulation of different types of ginsenosides in Panax ginseng cells. Acta Physiol Plant 38:1–8
Kang KB, Jayakodi M, Lee YS, Nguyen VB, Park HS, Koo HJ, Choi IY, Kim DH, Chung YJ, Ryu B, Lee DY, Sung SH, Yang TJ (2018) Identification of candidate UDP-glycosyltransferases involved in protopanaxadiol-type ginsenoside biosynthesis in Panax ginseng. Sci Rep. https://doi.org/10.1038/s41598-018-30262-7
Kiefer D, Pantuso T (2003) Panax ginseng. Am Fam Physician 68:1539–1542
Kim YS, Hahn EJ, Murthy HN, Paek KY (2004) Adventitious root growth and ginsenoside accumulation in Panax ginseng cultures as affected by methyl jasmonate. Biotechnol Lett 26:1619–1622
Kosová K, Prášil IT, Vítámvás P, Dobrev P, Motyka V, Floková K, Novák O, Turečková V, Rolčik J, Pešek B, Trávničková A, Gaudinová A, Galiba G, Janda T, Vlasáková E, Prášilová P, Vanková R (2012) Complex phytohormone responses during the cold acclimation of two wheat cultivars differing in cold tolerance, winter Samanta and spring Sandra. J Plant Physiol 169:567–576
Kumar S, Kaur G, Nayyar H (2008) Exogenous application of abscissic acid improves cold tolerance in chickpea (Cicer arietinum L.). J Agron Crop Sci 194:449–456
Lang V, Mantyla E, Welin B, Sundberg B, Palva ET (1994) Alterations in water status, endogenous abscisic acid content, and expression of rab18 gene during the development of freezing tolerance in Arabidopsis thaliana. Plant Physiol 104:1341–1349
Li D, Xu G, Ren G, Sun Y, Huang Y, Liu C (2017) The application of ultra-high-performance liquid chromatography coupled with a LTQ-Orbitrap mass technique to reveal the dynamic accumulation of secondary metabolites in licorice under ABA stress. Molecules. https://doi.org/10.3390/molecules22101742
Li J, Liu S, Wang J, Li J, Liu D, Li J, Gao W (2016) Fungal elicitors enhance ginsenosides biosynthesis, expression of functional genes as well as signal molecules accumulation in adventitious roots of Panax ginseng C. A. Mey. J Biotechnol 239:106–114
Liu J, Lan X, Bao R, Yuan Y, Wu S, Quan X (2019) Salicylic acid involved in chilling-induced accumulation of calycosin-7-O-β-D-glucoside in Astragalus membranaceus adventitious roots. Acta Physiol Plant 41:120
Lu J, Li JX, Wang SH, Yao L, Liang WX, Wang J, Gao WY (2018) Advances in ginsenoside biosynthesis and metabolic regulation. Biotechnol Appl Biochem 65:514–522
Maruyama K, Urano K, Yoshiwara K, Morishita Y, Sakurai N, Suzuki H, Kojima M, Sakakibara H, Shibata D, Saito K, Shinozaki K, Yamaguchi-Shinozaki K (2014) Integrated analysis of the effects of cold and dehydration on rice metabolites, phytohormones, and gene transcripts. Plant Physiol 164:1759–1771
Mehrotra R, Bhalothia P, Bansal P, Basantani MK, Bharti V, Mehrotra S (2014) Abscisic acid and abiotic stress tolerance-different tiers of regulation. J Plant Physiol 171:486–496
Murcia G, Fontana A, Pontin M, Baraldi R, Bertazza G, Piccoli PN (2017) ABA and GA3 regulate the synthesis of primary and secondary metabolites related to alleviation from biotic and abiotic stresses in grapevine. Phytochemistry 135:34–52
Murthy HN, Dandin VS, Paek KY (2014) Tools for biotechnological production of useful phytochemicals from adventitious root cultures. Phytochem Rev 15:1–17
Nagira Y, Ikegami K, Koshiba T, Ozeki Y (2006) Effect of ABA upon anthocyanin synthesis in regenerated torenia shoots. J Plant Res 119:137–144
Nazari M, Maali Amiri R, Mehraban FH, Khaneghah HZ (2012) Change in antioxidant responses against oxidative damage in black chickpea following cold acclimation. Russ J Plant Physiol 59:183–189
Ning C, Gao X, Wang C, Huo X, Liu Z, Sun H, Yang X, Sun P, Ma X, Meng Q, Liu K (2018) Hepatoprotective effect of ginsenoside Rg1 from Panax ginseng on carbon tetrachloride-induced acute liver injury by activating Nrf2 signaling pathway in mice. Environ Toxicol 33:1050–1060
Niranjana Murthy H, Dandin VS, Yoeup Paek K (2014) Hepatoprotective activity of ginsenosides from Panax ginseng adventitious roots against carbon tetrachloride treated hepatic injury in rats. J Ethnopharmacol 158:442–446
Oh JY, Kim YJ, Jang MG, Joo SC, Kwon WS, Kim SY, Jung SK, Yang DC (2014) Investigation of ginsenosides in different tissues after elicitor treatment in Panax ginseng. J Ginseng Res 38:270–277
Pareek A, Khurana A, Sharma AK, Kumar R (2017) An overview of signaling regulons during cold stress tolerance in plants. Curr Genom 18:498–511
Paul S, Shin HS, Kang SC (2012) Inhibition of inflammations and macrophage activation by ginsenoside-Re isolated from Korean ginseng (Panax ginseng C.A. Meyer). Food Chem Toxicol 50:1354–1361
Posmyk MM, Bailly C, Szafrańska K, Janas KM, Corbineau F (2005) Antioxidant enzymes and isoflavonoids in chilled soybean (Glycine max (L.) Merr.) seedlings. J Plant Physiol 162:403–412
Prasad TK, Anderson MD, Martin BA, Stewart CR (1994) Evidence for chilling-induced oxidative stress in maize seedlings and a regulatory role for hydrogen peroxide. Plant Cell 6:65–74
Rahimi S, Kim YJ, Devi BSR, Oh JY, Kim SY, Kwon WS, Yang DC (2015) Sodium nitroprusside enhances the elicitation power of methyl jasmonate for ginsenoside production in Panax ginseng roots. Res Chem Intermed 42:2937–2951
Ramakrishna A, Ravishankar GA (2011) Influence of abiotic stress signals on secondary metabolites in plants. Plant Signal Behav 6:1720–1731
Ruelland E, Vaultier MN, Zachowski A, Hurry V (2009) Cold signalling and cold acclimation in plants. Adv Bot Res 49:35–150
Sauter A, Davies WJ, Hartung W (2001) The long-distance abscisic acid signal in the droughted plant: the fate of the hormone on its way from root to shoot. J Exp Bot 52:1991–1997
Shen X, Zhao K, Liu L, Zhang K, Yuan H, Liao X, Wang Q, Guo X, Li F, Li T (2014) A role for PacMYBA in ABA-regulated anthocyanin biosynthesis in red-colored sweet cherry cv. Hong Deng (Prunus avium L.). Plant Cell Physiol 55:862–880
Song X, Wu H, Yin Z, Lian M, Yin C (2017) Endophytic bacteria isolated from Panax ginseng improves ginsenoside accumulation in adventitious ginseng root culture. Molecules. https://doi.org/10.3390/molecules22060837
Suzuki N, Mittler R (2006) Reactive oxygen species and temperature stress: a delicate balance between signalling and destruction. Physiol Plant 126:45–51
Tansakul P, Shibuya M, Kushiro T, Ebizuka Y (2006) Dammarenediol-II synthase, the first dedicated enzyme for ginsenoside biosynthesis, in Panax ginseng. FEBS Lett 580:5143–5149
Tewari RK, Paek KY (2011) Salicylic acid-induced nitric oxide and ROS generation stimulate ginsenoside accumulation in Panax ginseng roots. J Plant Growth Regul 30:396–404
Um Y, Lee Y, Kim SC, Jeong YJ, Kim GS, Choi DW, Cha SW, Kim OT (2017) Expression analysis of ginsenoside biosynthesis-related genes in methyl jasmonate-treated adventitious roots of Panax ginseng via DNA microarray analysis. Hortic Environ Biotechnol 58:376–383
Vishwakarma K, Upadhyay N, Kumar N, Yadav G, Singh J, Mishra RK, Kumar V, Verma R, Upadhyay RG, Pandey M, Sharma S (2017) Abscisic acid signaling and abiotic stress tolerance in plants: a review on current knowledge and future. Front Plant Sci. https://doi.org/10.3389/fpls.2017.00161
Wang Y, Li J, Wang J, Li Z (2010) Exogenous H2O2 improves the chilling tolerance of manilagrass and mascarenegrass by activating the antioxidative system. Plant Growth Regul 61:195–204
Wang J, Gao WY, Zuo BM, Liu H, Zhang LM, Huang LQ (2012) Gradually scale-up culture in a bioreactor promotes radical scavenging activity of Panax ginseng (C A. Meyer) adventitious roots on 1,1-diphenyl-2-picrylhydrazyl. Plant Growth Regul 67:101–105
Wang J, Gao W, Zuo B, Zhang L, Huang L (2013) Effect of methyl jasmonate on the ginsenoside content of Panax ginseng adventitious root cultures and on the genes involved in triterpene biosynthesis. Res Chem Intermed 39:1973–1980
Wang J, Wang S, Liu G, Edwards EJ, Duan W, Li S, Wang L (2016) The synthesis and accumulation of resveratrol are associated with veraison and abscisic acid concentration in Beihong (Vitis vinifera × Vitis amurensis) berry skin. Front Plant Sci. https://doi.org/10.3389/fpls.2016.01605
You J, Liu X, Zhang B, Xie Z, Hou Z, Yang Z (2015) Seasonal changes in soil acidity and related properties in ginseng artificial bed soils under a plastic shade. J Ginseng Res 39:81–88
Zhang M, Yuan B, Leng P (2009) The role of ABA in triggering ethylene biosynthesis and ripening of tomato fruit. J Exp Bot 60:1579–1588
Zhao J, Shi Z, Liu S, Li J, Huang W (2014a) Ginsenosides Rg1 from Panax ginseng: a potential therapy for acute liver failure patients? Evid-Based Complement Altern Med. https://doi.org/10.1155/2014/538059
Zhao S, Wang L, Liu L, Liang Y, Sun Y, Wu J (2014b) Both the mevalonate and the non-mevalonate pathways are involved in ginsenoside biosynthesis. Plant Cell Rep 33:393–400
Zhu M, Assmann SM (2017) Metabolic signatures in response to abscisic acid (ABA) treatment in Brassica napus guard cells revealed by metabolomics. Sci Rep. https://doi.org/10.1038/s41598-017-13166-w
Zhu JJ, Li YR, Liao JX (2013) Involvement of anthocyanins in the resistance to chilling-induced oxidative stress in Saccharum officinarum L. leaves. Plant Physiol Biochem 73:427–433
Acknowledgements
This study was supported by the Natural Science Foundation of Jilin Province of China (YD ZJ202101ZYTS197).
Author information
Authors and Affiliations
Contributions
JL and YY prepared materials and detected the ginsenosides Rg1 and Re. JL and WJ measured the ROS and ABA contents. SW performed gene expressions. XQ analyzed the data. SW and WW designed the experiments and wrote the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
Not applicable.
Additional information
Communicated by Ali R. Alan.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article belongs to the Topical Collection: Secondary Metabolites and Medicinal Plant Biotechnology.
Rights and permissions
About this article
Cite this article
Li, J., Yuan, Y., Jiang, W. et al. Abscisic acid is required for cold-induced accumulation of ginsenosides Rg1 and Re in Panax ginseng adventitious roots. Plant Cell Tiss Organ Cult 149, 325–333 (2022). https://doi.org/10.1007/s11240-021-02222-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11240-021-02222-z