Abstract
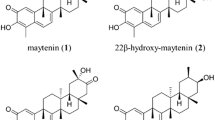
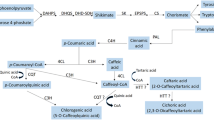
Quinonemethide triterpenes (QTs) are natural compounds that present anti-cancer action and are valuable chemotaxonomic markers in the Celastraceae plant family. However, the main obstacle to produce these compounds on a large scale is their low quantity in tissues of plants grown in situ. In addition, these molecules show a higher complex chemical structure and it is difficult to find strategies for enhancing their synthesis. In this context, the goal of this work was to evaluate the effect of inhibitors terbinafine, ancymidol, paclobutrazol, gibberellin (GA), and the association of GA + paclobutrazol on QT production from Monteverdia floribunda roots in vitro. After establishment of the total growth of the root system, the roots were subjected to the following inducing agents: ancymidol, uniconazole, paclobutrazol, GA, and terbinafine hydrochloride. Having established that in vitro roots from M. floribunda could produce QTs, the roots were inoculated in liquid WP medium with sodium acetate-1-13C and sodium acetate-2-13C. The results showed that ancymidol, GA, and the association of GA with paclobutrazol produced the best results obtained during QT elicitation. The growth regulator GA has a positive effect on the production of terpenoids from the mevalonate (MVA) pathway and it was confirmed that maytenin biosynthesis occurs via the MVA pathway.
Key message
The goal of this work was to evaluate the effect of inhibitors on quinonemethide triterpenes production from M. floribunda roots in vitro, besides the biosynthetic route was confirmed.



Similar content being viewed by others
References
Adam K-P, Zapp J (1998) Biosynthesis of the isoprene units of chamomile sesquiterpenes. Phytochemistry 48(6):953–959
Ahmad I, Kamran M, Ali S, Bilegjargal B, Cai T, Ahmad S, Meng X, Su W, Liu T, Han Q (2018) Uniconazole application strategies to improve lignin biosynthesis, lodging resistance and production of maize in semiarid regions. Field Crops Res 222:66–77
Bicalho KU, Santoni MM, Arendt P, Zanelli CF, Furlan M, Goossens A, Pollier J (2019) CYP712K4 catalyzes the C-29 oxidation of friedelin in the Maytenus ilicifolia quinone methide triterpenoid biosynthesis pathway. Plant Cell Physiol 60:11:2510–2522
Biral L, Simmons MP, Smidt EC, Tembrock LR, Bolson M, Archer RL, Lombardi JA (2017) Systematics of new world Maytenus (Celastraceae) and a new delimitation of the genus. Syst Bot 42(4):680–693
Biral L, Smidt EC, Bolson M, Lombardi JA (2015) A new species of Maytenus (Celastraceae) from the Brazilian Atlantic forest, with evidence of molecular phylogeny, and two new synonyms for Maytenus floribunda. Phytotaxa 231(1):53–62
Coolbaugh RC, Hirano SS, West CA (1978) Studies on the specificity and site of action of α-cyclopropyl-α-[p-methoxyphenyl]-5-pyrimidine methyl alcohol (ancymidol), a plant growth regulator. Plant Physiol 62(4):571–576
Coppede JS, Pina ES, Paz TA, Fachin AL, Marins MA, Bertoni BW, França SC, Pereira AMS (2014) Cell cultures of Maytenus ilicifolia Mart. are richer sources of quinone-methide triterpenoids than plant roots in natura. Plant Cell Tiss Organ Cult 118:33–43
Corsino J, De Carvalho PR, Kato MJ, Latorre LR, Oliveira OM, Araújo AR, Bolzani VD, França SC, Pereira AM, Furlan M (2000) Biosynthesis of friedelane and quinonemethide triterpenoids is compartmentalized in Maytenus aquifolium and Salacia campestris. Phytochemistry 55(7):741–748
Ferreira DF (2002) Sisvar: a computer statistical system. Ciênc Agrotec 2011(35):1039–1042
Flores-Sánchez IJ, Ortega-López J, del Montes-Horcasitas C, Ramos-Valdivia M AC (2002) Biosynthesis of sterols and triterpenes in cell suspension cultures of Uncaria tomentosa. Plant Cell Physiol 43(12):1502–1509
Fulton DC, Kroon PA, Threlfall DR (1994) Enzymological aspects of the redirection of terpenoid biosynthesis in elicitor-treated cultures of Tabernaemontana divaricata. Phytochemistry 35(5):1183–1186
Gunatilaka AAL, Fernando HC, Kikuchi T, Tezuka Y (1989) 1H and 13C NMR analysis of three quinone-methide triterpenoids. Magn Reson Chem 27:803–811
Henry M, Rahier A, Taton M (1992) Effect of gypsogenin 3,O-glucuronide pretreatment of Gypsophila paniculata and Saponaria officinalis cell suspension cultures on the activities of microsomal 2,3-oxidosqualene cycloartenol and amyrin cyclases. Phytochemistry 31(11):3855–3859
Hofmannová J, Schwarzerová K, Havelková L, Boríková P, Petrásek J, Opatrny Z (2008) A novel, cellulose synthesis inhibitory action of ancymidol impairs plant cell expansion. J Exp Bot 59(14):3963–3974
Inácio MC, Paz TA, Pereira AMS, Furlan M (2017) Endophytic Bacillus megaterium and exogenous stimuli affect the quinonemethide triterpenes production in adventitious roots of Peritassa campestris (Celastraceae). Plant Cell Tiss Organ Cult 131:15–26
Jaleel CA, Manivannan B, Sankar B, Kishorekumar A, Sankari S, Panneerselvam R (2007) Paclobutrazol enhances photosynthesis and ajmalicine production in Catharanthus roseus. Process Biochem 42(11):1566–1570
Jiang X, Wang Y, Xie H, Li R, Wei J, Liu Y (2019) Environmental behavior of paclobutrazol in soil and its toxicity on potato and taro plants. Environ Sci Pollut Res 26(26):27385–27395
Jones AMP, Saxena PK, Murch SJ (2009) Elicitation of secondary metabolism in Echinacea purpurea L. by gibberellic acid and triazoles. Eng Life Sci 9(3):205–2010
Mansouri H, Asrar Z, Amarowicz R (2011) The response of terpenoids to exogenous gibberellic acid in Cannabis sativa L. at vegetative stage. Acta Physiol Plant 33:1085–1091
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15:473–497
Naik PM, Al-Khayri JM (2016) Abiotic and biotic elicitors–role in secondary metabolites production through in vitro culture of medicinal plants, abiotic and biotic stress in plants—recent advances and future perspectives. IntechOpen. https://doi.org/10.5772/61442
Pavarini DP, Biasioli MMS, Moreira TMS, Magalhes LG, Andricopulo AD, Zanelli CF, Furlan M (2016) Combined “omics” techniques for targeted investigation on CYP450 roles during bioactive quinonemethide triterpenes biosynthesis: Maytenus spp. case study. Planta Med 82(01):S1–S381
Perassolo M, Quevedo CV, Busto VD, Giulietti AM, Talou JR (2011) Role of reactive oxygen species and proline cycle in anthraquinone accumulation in Rubia tinctorum cell suspension cultures subjected to methyl jasmonate elicitation. Plant Physiol Biochem 49(7):758–763
Pina ES, Silva DB, Teixeira SP, Coppede JS, Furlan M, França SC, Lopes NP, Pereira AM, Lopes AA (2016) Mevalonate-derived quinonemethide triterpenoid from in vitro roots of Peritassa laevigata and their localization in root tissue by MALDI imaging. Sci Rep 6:22627
Rademacher W (2000) Growth retardants: effects on gibberellin biosynthesis and other metabolic pathways. Annu Rev Plant Physiol Plant Mol Biol 51:501–531
Ramirez-Estrada K, Vidal-Limon H, Hidalgo D, Moyano E, Golenioswki M, Cusidó RM, Palazon J (2016) Elicitation, an effective strategy for the biotechnological production of bioactive high-added value compounds in plant cell factories. Molecules 21(2):182
Rohmer M, Seemann M, Horbach S, Bringer-Meyer S, Sahm H (1996) Glyceraldehyde 3-phosphate and pyruvate as precursors of isoprenic units in an alternative non-mevalonate pathway for terpenoid biosynthesis. J Am Chem Soc 118(11):2564–2566
Salazar GDCM, Silva GDF, De Sousa JR (1999) Chemical constituents from bark wood and leaves of Maytenus floribunda (Reiss). Acta Hort 501:205–208
Santos VAFFM, Dias NB, Teixeira SP, Palma MS, Furlan M (2021) Mapping biochemical pathways in Maytenus ilicifolia (Celastraceae) through integrated proteomics and histochemistry. J Braz Chem Soc 32:2: 237–248
Sharma D, Awasthi MD (2005) Uptake of soil applied paclobutrazol in mango (Mangifera indica L.) and its persistence in fruit and soil. Chemosphere 60(2):164–169
Shive JB, Sisler HD (1976) Effects of ancymidol (a growth retardant) and triarimol (a fungicide) on the growth, sterols, and gibberellins of Phaseolus vulgaris (L.). Plant Physiol 57(4):640–644
Singh H, Kumar S, Singh BD (2015) In vitro conservation of pointed gourd (Trichosanthes dioica) germplasm through slow-growth shoot cultures: effect of flurprimidol and triiodobenzoic acid. Sci Hort 182:41–46
Sugiura M, Kimura A, Fujiwara H (2006) Discrimination of enantiomers by means of NMR spectroscopy using chiral liquid crystalline solution: application to triazole fungicides, uniconazole and diniconazole. Magn Reson Chem 44(2):121–126
Suttle JC, Abrams SR, De Stefano-Beltrán L, Huckle LL (2012) Chemical inhibition of potato ABA-8’-hydroxylase activity alters in vitro and in vivo ABA metabolism and endogenous ABA levels but does not affect potato microtuber dormancy duration. J Exp Bot 63(15):5717–5725
Veeresham C, Mamatha R, Babu CP, Srisilam K, Kokate CK (2003) Production of taxol and its analogues from cell cultures of Taxus wallichiana. Pharm Biol 41(6):426–430
Xu LN, Zhao N, Chen JY, Ye PP, Nan XW, Zhou HH, Jiang QW, Yang Y, Huang JR, Yuan ML, Xing ZH, Wei MN, Li Y, Shi Z, Yan XJ (2019) Celastrol inhibits the growth of ovarian cancer cells in vitro and in vivo. Front Oncol 9:2
Zhang L, Luo Z, Cui S, Xie L, Yu J, Tang D, Ma X, Mou Y (2019) Residue of paclobutrazol and its regulatory effects on the secondary metabolites of Ophiopogon japonicas. Molecules 24(19):3504
Zhang N, Zhao B, Zhang HJ, Weeda S, Yang C, Yang ZC, Ren S, Guo YD (2013) Melatonin promotes water-stress tolerance, lateral root formation, and seed germination in cucumber (Cucumis sativus L.). J Pineal Res 54(1):15–23
Zhao J, Davis LC, Verpoorte R (2005) Elicitor signal transduction leading to production of plant secondary metabolites. Biotechnol Adv 23(4):283–333
Acknowledgements
The authors are grateful to the Biotechnology Unit (UNAERP). M.V. also thanks CAPES for the award of scholarships. The authors would like to thank Dr. Nivaldo Boralle (IQ/UNESP, Araraquara, SP, Brazil) for NMR analyses.
Author information
Authors and Affiliations
Contributions
The author’s contribution was changed: AMSP designed research; MV, BWB, EJC, LB and AAL: performed research; AMSP, BWB, AAL, and SCF analyzed data; AMSP and AAL: wrote the manuscript and all authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Communicated by Christophe Hano.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article belongs to the Topical Collection: Secondary Metabolites and Medicinal Plant Biotechnology.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Valdevite, M., Bertoni, B.W., Crevelin, E.J. et al. Modulation of quinonemethide triterpenes biosynthesis in Monteverdia floribunda (Reissek) biral root cultures by exogenous inhibitors. Plant Cell Tiss Organ Cult 149, 313–324 (2022). https://doi.org/10.1007/s11240-021-02214-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11240-021-02214-z