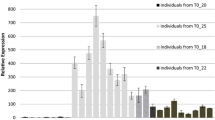
Abstract
The plasmid pBI121 cry1Ab used to transform peach explants to produce insect resistant plants was constructed by cloning the synthetic cryIAb gene with the intron of the castor bean catalase-1 gene into the pBI121 binary vector, under the control of the CaMV35S promoter. Leaf discs of peach (Prunus persica L.) were co-cultivated for two days with this construct in A. tumefaciens strain LBA 4404. Explants were plated on WPM medium supplemented with 125 mg L−1 kanamycin, 3% sucrose, 2.5 mg L−1 2,4-Dichlorophenoxyacetic acid, and 0.5 mg L−1 6-benzyladenine in darkness for callus formation. The calli were then selected on WPM medium supplemented with 125 mg L−1 kanamycin, 3% sucrose, 3.00 mg L−1 Thidiazuron, 1.0 mg L−1 kinetin, and 0.5 mg L−1 indoleacetic acid in the light for at least five subcultures (with a regeneration efficiency of 91.8%). The integration of the cry1Ab gene into the peach genome was confirmed by polymerase chain reaction (PCR) and northern blot analysis. A transformation efficiency of 28% was obtained when leaves were incubated for 15 min with A. tumefaciens. The cry1Ab gene expression was confirmed using RT-PCR, northern blot hybridization, immune-strip test, and insect bioassays, respectively. For insect bioassay, it was evident that the protein cryIAb expressed in transformed peach plants showed 100% mortality at 1000 ppm against Synanthedon exitiosa larvae after 96 h. The obtained results demonstrated a significant improvement of cryIAb toxin protein against lepidopteron larvae in peach.
Key message
In vitro peach regeneration from in vitro leaves is difficult, as well as regeneration using the method of genetic transfer such as agrobacterium to impart the desired characteristics of the plants with the aim of improvement is very difficult. This paper is the first to transgenic peach plants carrying cry1Ab gene for insect resistance particularly the peach tree borer.








Similar content being viewed by others
Abbreviations
- BA:
-
6-Benzyladenine
- bp:
-
Base pairs
- Cry:
-
Crystal protein
- IAA:
-
Indole-3-acetic acid
- IBA:
-
Indole-3-butyric acid
- 2iP:
-
N-6-(Δ2-isopentenyl) adenine
- Kn:
-
Kinetin
- TDZ:
-
Thiadiazuron (N-phenyl-N-1,2,3,-thiadiasol-5-ylurea)
- 2,4-D:
-
2,4-Dichlorophenoxyacetic acid
- WPM:
-
Woody plant medium
- MS:
-
Murashige and Skoog’s medium
- nptII:
-
Neomycin phosphotransferase
- PCR:
-
Polymerase chain reaction;
- RT-PCR:
-
Reverse transcriptase polymerase chain reaction
References
Abbasi H, Naderi R, Kafi M, Azadi P, Shakh-Asadi M, Okazaki K (2020) Effect of ‘Chloroxynil’on Agrobacterium-mediated transformation efficiency of Lilium cv ‘Manissa.’ Sci Hortic. https://doi.org/10.1016/j.scienta.2020.109404
Agdia, (2003) Bt-cryIA(b)/lA(c) immuno strip tests. Strip tests for the detection Bt- cryIA(b)/lA(c) protein. Agdia Incorporated, Elkhart, pp 1–3
Aisley PJ, Collins GG, Sedgley M (2001) Factors affecting Agrobacterium—mediated gene transfer and the selection of transgenic calli in paper shell almond (Prunus dulcis Mill.). J Hort Sci Biotechnol 76:522–528
Atia MA, Abdeldaym EA, Abdelsattar M, Ibrahim DS, Saleh I, Abd Elwahab M, Osman GH, Arif IA, Abdelaziz ME (2020) Piriformospora indica promotes cucumber tolerance against root-knot nematode by modulating photosynthesis and innate responsive genes. Saudi J Biol Sci 27:279–287
Balaraman K (2005) Occurrence and diversity of mosquitocidal strains of Bacillus thuringiensis. J Vector Borne Dis 42:81
Baranek J, Banaszak M, Lorent D, Kaznowski A, Konecka E (2020) Insecticidal activity of Bacillus thuringiensis Cry1, Cry2 and Vip3 toxin combinations in Spodoptera exigua control: highlights on synergism and data scoring. Entomol Gen 41:71
Betz FS, Hammond BG, Fuchs RL (2000) Safety and advantages of Bacillus thuringiensis-protected plants to control insect pests. Regul Toxicol Pharmacol 32:156–173
Bodh S, Singh D, Dogra RK, Chauhan N (2019) Comparative response of some peach [Prunus persica (L.) Batsch.] accessions for tree, foliage and floral traits. Int J Econ Plants 6:116–121
Butko P (2003) Cytolytic toxin of Cyt1A and its mechanism of membrane damage: data and hypotheses. Appl Environ Microbiol 69:2415–2422
CABI Crop Protection Compendium. (2014). Prunus persica (peach) datasheet. Retrieved from http://www.cabi.org/cpc/datasheet/44340.
Chen C, Wu JA (2012) Fast and sensitive quantitative lateral flow immunoassay for Cry1Ab based on a novel signal amplification conjugate. Sensors 12:11684–11696
Colby SM, Meredith CP (1990) Kanamycin sensitivity of cultured tissues of Vitis. Plant Cell Rep 9:237–240
Cosson V, Eschstruth A, Ratet P (2015) Medicago truncatula transformation using leaf explants. Agrobacterium protocols. Springer, New York, pp 43–56
Cottrell TE, Shapiro-Ilan DI (2006) Susceptibility of the peachtree borer, Synanthedon exitiosa, to Steinernema carpocapsae and Steinernema riobrave in laboratory and field trials. J Invertebr Pathol 92:85–88
Declerck V, Korban SS (1996) Influence of growth regulators and carbon sources on callus induction, growth and morphogenesis from leaf tissues of peach (Prunus persica L. Batsch). J Hortic Sci 71:49–55
Dulmage HT (1971) Production of delta-endotoxin by 18 isolates of Bacillus thuringiensis, serotype 3, in 3 fermentation media. J Invertebr Pathol 18:353–358
Dutton A, Romeis J, Bigler F (2005a) Effects of Bt maize expressing Cry1Ab and Bt spray on Spodoptera littoralis. Neth Entomol Soc 114:161–169
Dutton A, Romeis J, Bigler F (2005b) Effects of Bt maize expressing Cry1Ab and Bt spray on Spodoptera littoralis. Entomol Exp App 114:161–169
El-Gaied L, Mahmoud A, Salem R, Elmenofy W, Saleh I, Abulreesh HH, Arif IA, Osman G (2020) Characterization, cloning, expression and bioassay of vip3 gene isolated from an Egyptian Bacillus thuringiensis against whiteflies. Saudi J Biol Sci 27:1363–1367
Ferry N, Edwards MG, Gatehouse JA, Gatehouse AM (2004) Plant–insect interactions: molecular approaches to insect resistance. Curr Opin Biotechnol 15:155–161
Food and Agriculture Organization (2020) Retrieved from http://www.fao.org/faostat/en/#data/QC/visualize
Gentile A, Monticelli S, Damiano D (2002) Adventitious shoot regeneration in peach (Prunus persica L. Bastsch). Plant Cell Rep 20:1011–1016
Gentile A, Monticelli S, Damiano C (2003) Adventitious shoot regeneration for Agrobacterium-mediated transformation in Prunus. Acta Hortic 616:331–333
Gomez I, Sanchez J, Munoz-garay C, Matus V, Gill SS, Soberon M, Bravo A (2014) Bacillus thuringiensis Cry1A toxins are versatile proteins with multiple modes of action: two distinct pre-pores are involved in toxicity. Biochem J 459:383–396
Hao W, Li F, Yan W, Li C, Hao D (2017) Comparative metabolic profiling of four transgenic maize lines and two non-transgenic maize lines using high-performance liquid chromatography mass spectrometry. Acta Physiol Plant 39:1–10
Hofte H, Whiteley HR (1989) Insecticidal crystal proteins of Bacillus thuringiensis. Microbiol Rev 53:242–255
Jenkins JL, Dean DH (2000) Exploring the mechanism of action of insecticidal proteins by genetic engineering methods. In: Setlow JK (ed) Genetic engineering, vol 22. Springer, Boston
Johnson D, Cottrell T, Horton D (2005a) Lesser peachtree borer. Southeast Peach Growers Handb 1:270–272
Johnson D, Cottrell T, Horton D (2005b) Peachtree borer. In: Horton D, Johnson D (eds) Southeastern peach grower’s handbook. University of Georgia Cooperative Extension Service, Athens, pp 266–269
Kranthi KR, Stone GD (2020) Long-term impacts of Bt cotton in India. Nature Plants 6:188–196
Kumar M, Chimote V, Singh R, Mishra GP, Naik PS, Pandey SK, Chakrabarti SK (2010) Development of Bt transgenic potatoes for effective control of potato tuber moth by using cry1Ab gene regulated by GBSS promoter. Crop Prot 29:121–127
Lloyd G, McCown B (1980) Commercially-feasible micropropagation of mountain laurel, Kalmia latifolia, by use of shoot-tip culture. Proc Int Plant Prop Soc 30:421–427
Miguel CM, Druart P, Oliveira MM (1996) Shoot regeneration from adventitious buds induced on juvenile and adult almond (Prunus dulcis Mill.) explants. In Vitro Cell Dev Biol Plant 32:148–153
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15:473–479
Orbović V (2019) New developments in Agrobacterium-mediated transformation of tree fruit crops. Front Plant Sci 10:1253
Oropeza-Aburto A, Cervantes-Perez S, Albert VA, Herrera-Estrella L (2020) Agrobacterium tumefaciens mediated transformation of the aquatic carnivorous plant Utricularia gibba. Plant Method 16:1–11
Padilla IM, Golis A, Gentile A, Damiano C, Scorza R (2006) Evaluation of transformation in peach Prunus persica explants using green fluorescent protein (GFP) and beta-glucuronidase (GUS) reporter genes. Plant Cell Tiss Organ Cult 84:309–314
Pardo Lopez L, Soberon M, Bravo A (2013) Bacillus thuringiensis insecticidal three-domain Cry toxins: mode of action, insect resistance and consequences for crop protection. FEMS Microbiol Rev 37:3–22
Pasternak T, Prinsen E, Ayaydin F, Miskolczi P, Potters G, Asard H, Van Onckelen H, Dudits D, Fehér A (2002) The role of auxin, pH and stress in the activation of embryogenic cell division in leaf protoplast-derived cells of alfalfa (Medicago sativa L.). Plant Physiol 129:1807–1819
Pérez-Jiménez M, López-Soto MB, Cos-Terrer J (2013) In vitro callus induction from adult tissues of peach (Prunus persica L. Batsch). In Vitro Cell Dev Biol Plant 49:79–84
Petri C, Wang H, Alburquerque N, Faize M, Burgos L (2008) Agrobacterium-mediated transformation of apricot (Prunus armeniaca L.) leaf explants. Plant Cell Rep 27:1317–1324
Rahnama H, Nikmard M, Abolhasani M, Osfoori R, Sanjarian F, Habashi AA (2017) Immune analysis of cry1Ab-genetically modified potato by in-silico analysis and animal mode l. Food Sci Biotechnol 26:1437–1445
Ramirez-Romero R, Desneux N, Chaufaux J, Kaiser L (2008) Bt-maize effects on biological parameters of the non-target aphid Sitobion avenae (Homoptera: Aphididae) and Cry1Ab toxin detection. Pestic Biochem Physiol 91:110–115
Ranjekar PK, Patankar A, Gupta V, Bhatnagar R, Bentur J, Kumar PA (2003) Genetic engineering of crop plants for insect resistance. Curr Sci 84:321–329
Reilly CC, Gentry CR, McVay JR (1987) Biochemical evidence for resistance of rootstocks to the peachtree borer and species separation of peachtree borer and lesser peachtree borer (Lepidoptera: Sesiidae) on peach trees. J Econ Entomol 80:338–343
Reis RS, Vale EM, Sousa KR, Santa-Catarina C, Silveira V (2021) Pretreatment free of 2, 4-dichlorophenoxyacetic acid improves the differentiation of sugarcane somatic embryos by affecting the hormonal balance and the accumulation of reserves. Plant Cell Tissue Organ Cult 145:101–115
Ricci A, Capriotti L, Mezzetti B, Navacchi O, Sabbadini S (2020a) Adventitious shoot regeneration from in vitro leaf explants of the peach rootstock hansen 536. Plants 9:755
Ricci A, Sabbadini S, Prieto H, Padilla IM, Dardick C, Li Z, Scorza R, Limera C, Mezzetti B, Perez-Jimenez M, Burgos L (2020b) Genetic transformation in peach (Prunus persica L.): challenges and ways forward. Plants 9:971
Sabbadini S, Capriotti L, Molesini B, Pandolfini T, Navacchi O, Limera C, Ricci A, Mezzetti B (2019a) Comparison of regeneration capacity and Agrobacterium-mediated cell transformation efficiency of different cultivars and rootstocks of Vitis spp. via organogenesis. Sci Rep 9:1–10
Sabbadini S, Ricci A, Limera C, Baldoni D, Capriotti L, Mezzetti B (2019b) Factors affecting the regeneration, via organogenesis, and the selection of transgenic calli in the peach rootstock hansen 536 (Prunus persica × Prunus amygdalus) to express an RNAi construct against PPV virus. Plants 8:178
Salehian H, Rahnama H, Dezhsetan S, Babaei S (2021) Constitutive expression of a synthetic cry1Ab gene confers resistance to potato tuber moth (Phthorimaea operculella Zeller) larva. Crop Breed App Biotechnol 21(1):1–9
Sambrook J, Fritsch EF, Maniatis T (1989) Molecular cloning: a laboratory manual, 2nd edn. Cold spring harbor laboratory press
San B, Li Z, Hu Q, Reighard GL, Luo H (2014) Adventitious shoot regeneration from in vitro cultured leaf explants of peach rootstock Guardianfi is significantly enhanced by silver thiosulfate. Plant Cell Tiss Organ Cult 120:757–765
Shapiro-Ilan DI, Cottrell TE, Mizell RF, Horton DL, Davis J (2009) A novel approach to biological control with entomopathogenic nematodes: prophylactic control of the peach tree borer, Synanthedon exitiosa. Biol Control 48:259–263
Shapiro-Ilan DI, Cottrell TE, Mizell RF, Horton DL, Zaid A (2015) Field suppression of the peachtree borer, Synanthedon exitiosa, using Steinernema carpocapsae: Effects of irrigation, a sprayable gel and application method. Biol Control 82:7–12
Sidorova T, Mikhailov R, Pushin A, Miroshnichenko D, Dolgov S (2017) A non-antibiotic selection strategy uses the phosphomannose-isomerase (PMI) gene and green fluorescent protein (GFP) gene for Agrobacterium-mediated transformation of Prunus domestica L. leaf explants. Plant Cell Tiss Organ Cult 128:197–209
Sidorova T, Mikhailov R, Pushin A, Miroshnichenko D, Dolgov S (2019) Agrobacterium-mediated transformation of Russian commercial plum cv. “Startovaya” (Prunus domestica L.) with virus-derived hairpin RNA construct confers durable resistance to PPV infection in mature plants. Plant Sci 10:286
Soliman HI (2013) In vitro regeneration and genetic transformation of peach (Prunus persica L.) plants. Life Sci J 10:487–496
Soliman HI, Abo-El-Hasan F, El-seedy AS, Mabrouk YM (2017) Agrobacterium-mediated transformation and regeneration of transgenic tomato (Lycopersicon esculentum Mill.) plants using a synthetic cry1Ab gene for enhanced resistance against Tuta absoluta (Meyrick). J Microbiol Biotechnol Food Sci 7(1):67–74
Song GQ, Sink KC (2005) Optimizing shoot regeneration and transient expression factors for Agrobacterium tumefaciens transformation of sour cherry (Prunus cerasus L.) cultivar Montmorency. Sci Hortic 106:6
Song GQ, Prieto H, Orbovic V (2019) Agrobacterium-mediated transformation of tree fruit crops: methods, progress, and challenges. Front Plant Sci 10:226
Souza ADG, Chalfun NNJ, Faquin V, Souza AAD (2011) Production of peach grafts under hydroponic conditions. Ciênc Agrotecnol 35:322–326
Steel RG, Torrie JH, Dickey DA (1997) Principles and procedures of statistics a biometrical approach, 3rd edn. McGraw Hill Book Co., Inc., New York
Tabashnik BE, Cushing NL, Finson N, Johnson MW (1990) Field development of resistance to Bacillus thuringiensis in diamondback moth (Lepidoptera: Plutellidae). J Econ Entomol 83:1671–1676
Tanaka A, Mita S, Ohta S, Kyozuka J, Shimamoto K, Nakamura K (1990) Enhancement of foreign gene expression by a dicot intron in Rice but not in tobacco is correlated with an increased level of mRNA and an efficient splicing of the intron. Nucl Acid Res 18:6767–6770
Thirukkumaran G, Ntui VO, Khan RS, Mii M (2009) Thidiazuron: an efficient plant growth regulator for enhancing Agrobacterium-mediated transformation in Petunia hybrida. Plant Cell Tiss Organ Cult 99:109–115
Wang Y, Georgi LL, Reighard GL, Scorza R, Abbott AG (2002) Genetic mapping of the evergrowing gene in peach [Prunus persica (L.) Batsch]. J Hered 93:352–358
Wang K, Liu H, Du L, Ye X (2017) Generation of marker -free transgenic hexaploid wheat via an Agrobacterium -mediated co-transformation strategy in commercial Chinese wheat varieties. Plant Biotechnol J 15(5):614–623
Wilcox DR, Shivakumar AG, Melin BE, Miller MF, Benson TA, Schopp CW, Casuto D, Gundling GJ, Bolling TJ, Spear BB, Fox JL (1986) Genetic engineering of bioinsecticides, vol 395. Academic Press, New York
Xu C, Cheng J, Lin H, Lin C, Gao J, Shen Z (2018) Characterization of transgenic rice expressing fusion protein Cry1Ab/Vip3A for insect resistance. Sci Rep 8:1–8
Xu S, Lai E, Zhao L, Cai Y, Ogutu C, Cherono S, Han Y, Zheng B (2020) Development of a fast and efficient root transgenic system for functional genomics and genetic engineering in peach. Sci Rep 10:1–9
Ye GY, Shu QY, Yao HW, Cui HR, Cheng XY, Hu C, Xia YW, Gao MW, Altosaar I (2001) Field evaluation of resistance of transgenic rice containing a synthetic cry1Ab gene from Bacillus thuringiensis Berliner to stem borers. J Econ Entomol 94:271–276
Zhang BH, Liu F, Liu ZH, Wang HM, Yao CB (2001) Effects of kanamycin on tissue culture and somatic embryogenesis in cotton. Plant Grow Regul 33:137–149
Zhou H, Li M, Zhao X, Fan X, Guo A (2010) Plant regeneration from in vitro leaves of the peach rootstock ‘Nemaguard’(Prunus persica × P. davidiana). Plant Cell Tiss Organ Cult 101:79–87
Zuo JR, Niu QW, Frugis G, Chua NH (2002) The wuschel gene promotes vegetative-to-embryonic transition in Arabidopsis. Plant J 30:349–359
Acknowledgements
The authors are thankful to the Tissue culture and Biotechnology Labs., Mariout Research Station, Desert Research Center, Egypt.
Author information
Authors and Affiliations
Contributions
NK planned the whole work, performed the tissue culture experiments, helped write the draft and designed the figures and tables. EM analyzed data, helped in the write, participated in the discussion and results, contributed in the statistical analysis, and reviewed several drafts of the manuscript. HS conceived the research, performed the experiments, provided lab facilities, performed molecular analysis, and submitted the MS. WA helped in experimental accomplishment, reviewed and edited the manuscript. All authors contributed to the final manuscript. All authors read and approved the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interests. The authors do not have any competing financial interest or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Communicated by Heidi Halbwirth.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kadasa, N.M., Metwali, E.M.R., Soliman, H.I.A. et al. Creation of borer pests resistance genetically engineering peach (Prunus persica L.) plants by constitutively overexpressing the cry1Ab gene. Plant Cell Tiss Organ Cult 148, 465–477 (2022). https://doi.org/10.1007/s11240-021-02198-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11240-021-02198-w