Abstract
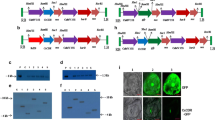
The putative transgenic mungbean (Vigna radiata Var. OBGG-52) plants were regenerated on Murashige and Skoog (1962) medium supplemented with 1.0 mg/L 6-benzylaminopurine (BA). The transgenic status of transformants (T2 generation) was determined through PCR analysis using nptII and ICE1 gene-specific primers as well as uidA reporter gene expression. About 75% of the transformed plantlets were successfully established in the greenhouse. Overexpression of ICE1 gene in transgenic mungbean plants resulted in cold-tolerance at the seedling stage when compared to non-transformed plants. Seeds from T2 generation were further studied for cold tolerance through nutrient culture. Transgenic seeds had significantly increased germination and growth on a nutrient medium at 10–14 °C in growth chamber compared to the non-transgenic control. Moreover, the root and shoot length of transformed plants were significantly increased at 10–14 °C as compared with the non-transformed plants. Under cold stress, it was also observed that the transformed plantlets have significantly higher chlorophyll, proline, and lipid content as well as anti oxidative enzyme activity as compared to non-transformed plants. This study will help in mungbean improvement program for abiotic stress tolerance.
Key message
Cold temperature damaged the crop productivity of mungbean (Vigna radiata L. Wilczek), a high protein rich legume crop. The overexpression of cold responsive transcription factor, INDUCER OF CBF EXPRESSION 1 (ICE1) in mungbean enhanced cold tolerance.








Similar content being viewed by others
References
Aebi H (1984) Catalase in vitro. Methods in enzymology, vol 105. Academic Press, New York, pp 121–126
Anandan R, Prakash M, Sunilkumar B, Deanathayalan T (2019) In vitro transverse section thin cell layer culture in mungbean (Vigna radiata (L.) Wilczek. Indian J Exp Biol 57:324–329
Atif RM, Patat-Ochatt EM, Svabova L, Ondrej V, Klenoticova H, Jacas L, Griga M, Ochatt SJ (2013) Gene transfer in legumes. In: Luttge U, et al. (eds) Progress in botany 74. Springer-Verlag, Berlin, Heidelberg, pp 37–91. https://doi.org/10.1007/978-3-642-30967-0_2
Avenido RA, Hattori K (2001) Benzyladenine-preconditioning in germinating mung bean seedlings stimulates axillary buds in cotyledonary nodes resulting in multiple shoot regeneration. Breed Sci 51:137–142
Baek KH, Skinner DZ (2003) Alteration of antioxidant enzyme gene expression during cold acclimation of near-isogenic wheat lines. Plant Sci 165:1221–1227
Bates LS, Waldren RP, Tare ID (1973) Rapid determination of free proline for water-stress studies. Plant Soil 39:205–207
Beauchamp C, Fridovich I (1971) Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Ann Biochem 44:128–143
Chandra A, Pental D (2003) Regeneration and genetic transformation of grain legumes: an overview. Curr Sci 84:381–387
Chaudhary D, Madanpotra S, Jaiwal R, Saini A, Kumar P, Jaiwal PK (2007) Agrobacterium tumefaciens-mediated high frequency genetic transformation of an Indian cowpea (Vigna unguiculata L. Walp) cultivar and transmission of transgene into progeny. Plant Sci 172:692–700
Chinnusamy V, Zhu J, Zhu JK (2007) Cold stress regulation of gene expression in plants. Trends Plant Sci 12:444–451
Chinnusamy V, Zhu JK, Sunkar R (2010) Gene regulation during cold stress acclimation in plants. Methods Mol Biol 639:39–55. https://doi.org/10.1007/978-1-60761-702-0_3
Chinnusamy V, Ohta M, Kanrar S, Lee BH, Hong X, Agarwal M, Zhu JK (2003) ICE1: a regulator of cold-induced transcriptome and freezing tolerance in Arabidopsis. Genes Dev 17(8):1043–1054
Doyle JJ, Doyle JL (1987) A rapid DNA isolation procedure for small quantities of fresh leaf tissue. Phytochem Bull 19:11–15
Dubios MK, Gilles JK, Robers PA, Smith F (1951) Calorimetric determination of sugar and related substance. Anal Chem 26:351–356
Eapen S (2008) Advances in development of transgenic pulse crops. Biotechnol Adv 26:162–168
Fowler S, Thomashow MF (2002) Arabidopsis transcriptome profiling indicates that multiple regulatory pathways are activated during cold acclimation in addition to the CBF cold response pathway. Plant Cell 14:1675–1690
Frankow-Lindberg BE (2001) Adaptation to winter stress in nine white clover populations: changes in non-structural carbohydrates during exposure to simulated winter conditions and ‘spring’ re-growth potential. Ann Bot 88:745–751
Gilmour SJ, Zarka DG, Stockinger EJ, Salazar MP, Houghton JM, Thomashow MF (1998) Low temperature regulation of the Arabidopsis CBF family of AP2 transcriptional activators as an early step in cold-induced COR gene expression. Plant J 16:433–442
Hara M, Terashima S, Fukaya T, Kuboi T (2003) Enhancement of cold tolerance and inhibition of lipid peroxidation by citrus dehydrin in transgenic tobacco. Planta 217(2):290–298
Hernández-Nistal J, Dopico B, Labrador E (2002) Cold and salt stress regulates the expression and activity of a chickpea cytosolic Cu/Zn superoxide dismutase. Plant Sci 163:507–514
Jaiwal PK, Kumari R, Ignacimuthu S, Potrykus I, Sautter C (2001) Agrobacterium tumefaciens-mediated genetic transformation of mungbean (Vigna radiata L. Wilczek)—a recalcitrant grain legume. Plant Sci 161:239–247
Jefferson RA, Kavanagh TA, Bevan MW (1987) GUS fusions: β-glucorodinase as a sensitive and versatile gene fusion marker in higher plants. The EMBO 6:3901–3907
Jha P, Shashi AA, Rustagi A, Agnihotri PK, Kulkarni VM, Bhat V (2011) Efficient Agrobacterium-mediated transformation of Pennisetum glaucum (L.) R. Br. using shoot apices as explants source. Plant Cell Tissue Organ Cult 107:501–512
Jing JF, Gao Q, Chen Y, Wang JH (2005) Transfer of Arabidopsis CBF1 gene leads to increased proline content in rice plants. Chin J Cell Biol 27:73–76
Juan JX, Yu XH, Jiang XM, Gao Z, Zhang Y, Li W, Duan YD, Yang G (2015) Agrobacterium-mediated transformation of tomato with the ICE1 transcription factor gene. Genet Mol Res 14:597–608
Kleinbaum D, Kupper LL, Muller KE, Nizam A (1998) Applied regression analysis and other multivariable methods, 3rd edn. Duxbury Press, Crawfordsville, Indiana, pp 136–159
Ko TS, Korban SS (2004) Enhancing the frequency of somatic embryogenesis following Agrobacterium-mediated transformation of immature cotyledon of soybean (Glycine max (L.) Merrill). In Vitro Cell Dev Biol Plant 40:552–558
Krishanamurthy KV, Suhasini K, Sagare AP, Meixner M, deKathen PT, Schieder O (2000) Agrobacterium-mediated transformation of chickpea (Cicer arietinum L.) embryo-axes. Plant Cell Rep 19:235–240
Kubien DS, von Caemmerer S, Furbank RT, Sage RF (2003) C4 photosynthesis at low temperature. A study using transgenic plants with reduced amounts of rubisco. Plant Physiol 132:1577–1585
Lambrides CJ, Godwin ID (2007) Mungbean. Pulses, sugar and tuber crops. Springer, Berlin, Heidelberg, pp 69–90
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25:402–408
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein analysis. J Biol Chem 193:265–271
Mahalakshmi SL, Leela T, Manoj Kumar S (2006) Enhanced genetic efficiency of mungbean by use of primary leaf explants. Curr Sci 91:93–98
Mundhara R, Rashid A (2006) Recalcitrant grain legume Vigna radiata, mung bean, made to regenerate on change of hormonal and cultural conditions. Plant Cell Tissue Organ Cult 85:265–270
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Planta 15:473–497
Muruganantham M, Amutha S, Selvaraj N, Vengadesan G, Ganapathi A (2007) Efficient Agrobacterium-mediated transformation of Vigna mungo using immature cotyledonary node explants and phosphinothricin as the selection agent. In Vitro Cell Dev Biol Plant 43:550–557
Nair RM, Yang RY, Easdown WJ, Thavarajah D, Thavarajah P, Hughes JDA, Keatinge JDH (2013) Biofortification of mungbean (Vigna radiata) as a whole food to enhance human health. J Sci Food Agric 93:1805–1813
Nayyar H, Chander K, Kumar S, Bains T (2005) Glycine betaine mitigates cold stress damage in Chickpea. Agron Sustain Dev 25:81–388
Sahoo DP, Kumar S, Mishra S, Kobayashi Y, Panda SK, Sahoo L (2016) Enhanced salinity tolerance in transgenic mungbean over-expressing Arabidopsis antiporter (NHX1) gene. Mol Breed 36:144–154
Saini R, Jaiwal PK (2007) Agrobacterium tumefaciens—mediated transformation of black gram: an assessment of factors influencing the efficiency of uidA gene transfer. Biol Plant 51:69–74
Sharma KK, Anjaiah V (2000) An efficient method for the production of transgenic plants of peanut (Arachis hypogaea L.) through Agrobacterium tumefaciens-mediated genetic transformation. Plant Sci 159:7–19
Shinozaki K, Yamaguchi-Shinozaki K (2000) Molecular responses to dehydration and low temperature: differences and cross-talk between two stress signalling pathways. Curr Opin Plant Biol 3:217–223
Sonia Saini R, Singh RP, Jaiwal PK (2007) Agrobacterium tumefaciens mediated transfer of Phaseolus vulgaris α-amylase inhibitor-1 gene into mungbean (Vigna radiata (L.) Wilczek) using bar as selectable marker. Plant Cell Rep 26:187–198
Srinivasan T, Verma VK, Kirti PB (2004) Efficient shoot regeneration in pigeon pea, Cajanuscajan (L.) Mill sp. using seedling petioles. Curr Sci 86:30–32
Steponkus PL (1984) Role of the plasma membrane in freezing injury and cold acclimation. Annu Rev Plant Physiol 35:543–584
Steponkus PL, Uemura M, Webb MS (1993) A contrast of the cryostability of the plasma membrane of winter rye and spring oat-two species that widely differ in their freezing tolerance and plasma membrane lipid composition. In: Steponkus PL (ed) Advances in low-temperature biology, vol 2. JAI Press, London, pp 211–312
Suzuki K, Nagasuga K, Okada M (2008) The chilling injury induced by high root temperature in the leaves of rice seedlings. Plant Cell Physiol 49:433–442
Svabova L, Griga M (2008) The effect of co-cultivation treatments on transformation efficiency in pea (Pisum sativum L.). Plant Cell Tissue Organ Cult 95:293–304
Thomashow MF (1999) Plant cold acclimation: freezing tolerance genes and regulatory mechanisms. Annu Rev Plant Physiol 50:571–599
Valizadeh-Kamrana R, Toorchia M, Mogadama M, Mohammadic H, Pessaraklid M (2018) Effects of freeze and cold stress on certain physiological and biochemical traits in sensitive and tolerant barley (Hordeum vulgare) genotypes. J Plant Nutr 41:102–111
Vernon LP (1960) Spectrophotometric determination of chlorophyllsand heophytins in plant extracts. Anal Chem 32:1144–1150
Vijayan S, Kirti PB (2012) Mungbean plants expressing BjNPR1 exhibit enhanced resistance against the seedling rot pathogen, Rhizoctonia solani. Transgenic Res 21:193–200
Vijayan S, Beena MR, Kirti PB (2006) Simple and effective regeneration of mungbean (Vigna radiata (L) Wilczek.) using cotyledonary node explants. J Plant Biochem Biotechnol 15:131–134
Welti R, Li W, Li M, Sang Y, Biesiada H, Zhou HE, Rajashekar CB, Williams TD, Wang X (2002) Profiling membrane lipids in plant stress responses. Role of phospholipase Da in freezing induced lipid changes in Arabidopsis. J Biol Chem 277:31994–32002
Williams WP (1990) Cold-induced lipid phase transitions. Philos Trans R Soc Lond B 326:555–570
Wu J, Liu C, Seng S, Khan MA, Sui J, Gong B, Liu C, Wu C, Zhong X, He J, Yi M (2015) Somatic embryogenesis and Agrobacterium-mediated transformation of Gladiolus hybridus cv. advance red. Plant Cell Tissue Organ Cult 120:717–728
Xiang DJ, Hu XY, Zhang Y, Yin KD (2008) Over-expression of ICE1 gene in transgenic rice improves cold tolerance. Rice Sci 15(3):173–178
Yadav SK, Sreenu P, Maheswari M, Vanaja M, Venkateswarlu B (2010) Efficient shoot regeneration from double cotyledonary node explants of green gram (Vigna radiata L. Wilczek). Indian J Biotechnol 9:403–407
Yadav SK, Katikala S, Yellisetty V, Kannepalle A, Narayana JL, Maddi V, Maheswari Mandapaka M, Shanker AK, Bandi V, Kirti PB (2012) Optimization of Agrobacterium mediated genetic transformation of cotyledonary node explants of Vigna radiata. Springer Plus 1:59. https://doi.org/10.1186/2193-1801-1
Yamada T, Teraishi M, Hattori K, Ishimoto M (2001) Transformation of azuki bean by Agrobacterium tumefaciens. Plant Cell Tissue Organ Cult 64:47–54
Yuan HM, Sheng Y, Chen WJ, Lu YQ, Tang X, Ou-Yang M, Huang X (2017) Overexpression of Hevea brasiliensis HbICE1 enhances cold tolerance in Arabidopsis. Front Plant Sci 8:1462
Zuo ZF, Kang HG, Park MY, Jeong H, SunHJ YDH, Lee YE, Song PS, Lee HY (2019) Overexpression of ICE1, a regulator of cold-induced transcriptome, confers cold tolerance to transgenic Zoysia japonica. J Plant Biol 62(2):137–146
Acknowledgements
The authors wish to acknowledge to Department of Biotechnology, Govt. of India for providing infrastructural facility and fund for research and FIST grant, DST, Govt. of India. The authors wish to acknowledge to Dr. L. Sahoo, Department of Biosciences and Bioengineering, Indian Institute of Technology, Guwahati providing ICE1gene construct for the experiment.
Author information
Authors and Affiliations
Contributions
GRR & SKP design the experiment, DS, RGS & KRJ conducted the experiments and data preparation, GRR prepared the final draft and AB and SKP have fully edited the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Communicated by Sergio J. Ochatt.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Rout, G.R., Bansal, A., Swain, D. et al. Overexpression of ICE1 gene in mungbean (Vigna radiata L.) for cold tolerance. Plant Cell Tiss Organ Cult 143, 593–608 (2020). https://doi.org/10.1007/s11240-020-01944-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11240-020-01944-w