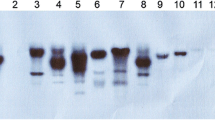
Abstract
Potato crops are particularly vulnerable to weed competition from emergence to canopy closure and are subject to significant yield loss. Glyphosate is broad spectrum herbicide used to control weeds worldwide. In order to incorporate glyphosate resistant trait in four potato cultivars (Lady Olympia, Desiree, Agria and Granola), an efficient, cost effective, reproducible and stable Agrobacterium-mediated genetic transformation protocol was performed using leaf and internodal explants. Agrobacterium strain LBA4404 harboring newly modified recombinant binary vector pCAMHE-EPSPS containing EPSP synthase gene under the control of Cauliflower mosaic virus 35S promoter was used to infect explants. The overall transformation efficiency was 26.4%. Of the 280 plants transferred to greenhouse, 74 plants were found to be PCR positive with gene of interest. GUS histochemical, Southern blot, RT-qPCR, lateral flow dipstick assays confirmed integration and expression of EPSPS in primary transformants. The putative transgenic plants developed from these cultivars possessed enhanced resistance to glyphosate applications in T0 and first tuber generation. These transgenic potato lines could be used as source of germplasm for an efficient potato breeding program.
Key message
The article reports the development of potato transgenic lines with enhanced tolerance against glyphosate.







Similar content being viewed by others
References
Aldemita RR, Reaño IM, Solis RO, Hautea RA (2015) Trends in global approvals of biotech crops (1992–2014). GM Crops Food 6:150–166
Americanos P (1994) Weed management in potatoes, weed management for developing Countries. In: Caseley RJ, Parker C (eds) Labrada. FAO, Rome, pp 295–300
Amiri AN, Bakhsh A (2019) An effective pest management approach in potato to combat insect pests and herbicide. 3 Biotech 9:16. https://doi.org/10.1007/s13205-018-1536-0
Anayol E, Bakhsh A, Karakoç ÖC, Onarıcı S, Köm D, Aasim M, Özcan SF, Barpete S, Khabbazi SD, Önol B, Sancak C (2016) Towards better insect management strategy: restriction of insecticidal gene expression to biting sites in transgenic cotton. Plant Biotechnol Rep 10:83–94
Bagri DS, Upadhyay DC, Jain SK, Upadhyay CP (2018) Biotechnological improvement of nutritional and therapeutic value of cultivated potato. Front Biosci 10:217–228
Bakhsh A, Siddiq S, Husnain T (2012) A molecular approach to combat spatio-temporal variation in insecticidal gene (Cry1Ac) expression in cotton. Euphytica 183:65–74
Bakhsh A, Baloch FS, Hatipöğlu R, Ozkan H (2015) Use of genetic engineering, benefits and health concerns. In: Hui YH, Özgül Evranuz E (eds) Handbook of Vegetable Preservation and Processing, 2nd edn. CRC Press, Boca Raton, pp 81–112
Banerjee AK, Prat S, Hannapel DJ (2006) Efficient production of transgenic potato (S. tuberosum L. ssp. andigena) plants via Agrobacterium tumefaciens-mediated transformation. Plant Sci 170:732–738
Beaujean A, Sangwan R, Lecardonnel A, Sangwan-Norreel B (1998) Agrobacterium-mediated transformation of three economically important potato cultivars using sliced internodal explants, an efficient protocol of transformation. J Exp Bot 49:1589–1595
Brookes G, Barfoot P (2011) Global impact of biotech crops, environmental effects 1996–2008. AgBioforum 13(1):76–94. http://www.agbioforum.org
Brookes G, Barfoot P (2014) Economic impact of GM crops: the global income and production effects 1996-2012. GM Crops 5:1–11. https://doi.org/10.4161/gmcr.28098
Brown TA (2001) Southern blotting and related DNA detection techniques/encyclopedia of life sciences. Wiley, Chichester
Colquhoun JB, Konieczka CM, Rittmeyer RA (2009) Ability of potato cultivars to tolerate and suppress weeds. Weed Technol 23:287–291
Conley SP, Binning LK, Connell TR (2001) Effect of cultivar, row spacing, and weed management on weed biomass, potato yield, and net crop value. Am J Potato Res 78:31–37
Dale PJ, Hampson KK (1995) An assessment of morphogenic and transformation efficiency in a range of varieties of potato (Solanum tuberosum L.). Euphytica 85:101–108
De Block M (1988) Genotype-independent leaf disc transformation of potato (Solanum tuberosum) using Agrobacterium tumefaciens. Theor Appl Genet 76:767–774
Dill G (2005) Glyphosate resistant crops: history, status and future. Pest Manag Sci 61:219–224
Ducreux LMJ, Morris WL, Taylor MA, Millam S (2005) Agrobacterium mediated transformation of Solanum phureja. Plant Cell Rep 24:10–14
FAOSTAT data (2017) http://www.fao.org/home/en/ Accessed on 30 July, 2018
Felix J, Ivany J, Kegode GO, Doohan D (2008) Timing potato cultivation using weedcast model. Weed Sci. https://doi.org/10.1614/WS-08-019.1
Figueira E, Figueiredo L, MonteNeshich D (1994) Transformation of potato (Solanum tuberosum) cv. Mantiqueira using Agrobacterium tumefaciens and evaluation of herbicide resistance. Plant Cell Rep 3:666–670
Göre ME (2017) Fungal seed borne pathogens infecting potato seed tubers from Turkey 2011–2014. J Plant Dis Protect 124:539–551
Green JM (2012) The benefits of herbicide-resistant crops. Pest Manag Sci 68:1323–1331
Greenbaum D, Colangelo C, Williams K, Gerstein M (2003) Comparing protein abundance and mRNA expression levels on a genomic scale. Genome Biol 4:117
Halterman D, Guenthner J, Collinge S, Butler N, Douches D (2016) Biotech potatoes in the 21st century: 20 years since the first biotech potato. Am J Potato 93:1–20
Hussain T, Aksoy E, Caliskan ME, Bakhsh A (2019) Transgenic potato lines expressing hairpin RNAi construct of molting-associated EcR gene exhibit enhanced resistance against Colorado potato beetle (Leptinotarsa decemlineata, Say). Transgenic Res 28:1–14. https://doi.org/10.1007/s11248-018-0109-7
Imran M, Asad S, Barboza AL, Galeano E, Carrer H, Mukhtar Z (2017) Genetically transformed tobacco plants expressing synthetic EPSPS gene confer tolerance against glyphosate herbicide. Physiol Mol Biol Plants 23:453–460
ISAAA (2016) Global status of commercialized Biotech/GM crops. ISAAA Brief No. 52. ISAAA:Ithaca, NY
ISAAA (2019) Potato (Solanum tubersom L.). GM events. http://www.isaaa.org/gmapprovaldatabase/advsearch/default.asp?CropID=16&TraitTypeID=1&DeveloperID=22&CountryID=US&ApprovalTypeID=Any. Accessed 7 Jan 2017
James C (2013) Global status of commercialized biotech/GM crops. ISAAA Brief No. 46 ISAAA, Ithaca, NY
Khan GA, Bakhsh A, Riazuddin S, Husnain T (2011) Introduction of cry1Ab gene into cotton (Gossypium hirsutum) enhances resistance against lepidopteran pest (Helicoverpa armigera). Span J Agric Res 9:296–300
Kumar A (1995) Agrobacterium-mediated transformation of potato genotypes. In: Gartland KMA, Davey MR (eds) Methods in molecular biology, Agrobacterium protocols, 44. Humana Press, Totowa, pp 121–128
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real time quantitative PCR and the 2ΔΔC(T) method. Methods 25:402–408
Maqbool A, Abbas W, Rao AQ, Irfan M, Zahur M, Bakhsh A, Riazuddin S, Husnain T (2010) Gossypium arboreum GHSP26 enhances drought tolerance in Gossypium hirsutum. Biotechnol Prog 26:21–25
Murashige T, Skoog F (1962) A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol Plant 15:473–497
Nain V, Jaiswal R, Dalal M, Ramesh B, Kumar A (2005) Polymerase chain reaction analysis of transgenic plants contaminated by Agrobacterium. Plant Mol Biol Rep 23:59–65
Nicolia A, Manzo A, Veronesi F, Rosellini D (2014) An overview of the last 10 years of genetically engineered crop safety research. Crit Rev Biotechnol 34:77–88
Nicot N, Hausman JF, Hoffman L, Evers D (2005) Housekeeping gene selection for real time PCR normalization in potato during biotic and abiotic stress. J Exp Bot 56:2907–2914
Oxtoby E, Hughes MA (1990) Engineering herbicide tolerance into crops. Trends Biotechnol 8:61–65
Padegimas L, Shulga OA, Skryabin KG (1994) Herbicide phosphinothricin tolerance in transgenic plants Nicotiana tabacum and Solanum tuberosum. Mol Biol 28:437–443
Peixoto FP, Gomes-Laranjo J, Vicente JA, Madeira VMC (2008) Comparative effects of the herbicides dicamba, 2,4-D and paraquat on non-green potato tuber calli. J Plant Physiol 165:1125–1133
Posthuma-Trumpie GA, Korf J, van Amerongen A (2009) Lateral flow (immuno) assay: its strengths, weaknesses, opportunities and threats A literature survey. Anal Bioanal Chem 393(2):569–582
Rao CK (2005) Transgenic Bt technology 3, expression of transgenes. http://www.fbae.org/2009/FBAE/website/special-topics_views_transgenic_bt_technology3.html
Rao AQ, Bakhsh A, Nasir IA, Riazuddin S, Husnain T (2011) Phytochrome B mRNA expression enhances biomass yield and physiology of cotton plants. Afr J Biotechnol 10:1818–1826
Sahoo KK, Tripathi AK, Pareek A, Sopory SK, Singla-Pareek SL (2011) An improved protocol for efficient transformation and regeneration of diverse indica rice cultivars. Plant Methods 7:49
Sambrook J, Russell D (2001) Molecular cloning: a laboratory manual, 3rd edn. Cold Spring Harbor Laboratory Press, Cold Spring Harbor
Soto N, Enriquez GA, Ferreira A, Corrada M, Fuentes A, Tiel K, Pujol M (2007) Efficient trans-formation of potato stem segments from cv. Desiree using phosphinothricin as selection marker. Biotechnol Applicada 24:139–144
Southern EM (1975) Detection of specific sequence among DNA fragments separated by gel electrophoresis. J Mol Biol 98:503–517
Tripathi B, Singh CM, Bhargava M (1989) Comparative efficacy of herbicides in potato under conditions of north-western Himalayas. Pesticides 23:37–38
Üremiş İ, Caliskan ME, Uludağ A, Caliskan S (2009) Weed management in early-season potato production in the Mediterranean conditions of Turkey. Bulg J Agric Sci 15:423–434
Veale MA, Slabbert MM, Van Emmenes L (2012) Agrobacterium-mediated transformation of potato cv. Mnandi for resistance to the potato tuber moth (Phthorimaea operculella). S Afr J Bot 80:67–74
Wang Q, Xing S, Pan Q, Yuan F, Zhao J, Tian Y, Chen Y, Wang G, Tang K (2012) Development of efficient Catharanthus roseus regeneration and transformation system using Agrobacterium tumefaciens and hypocotyls as explants. BMC Biotechnol 12:34
Yang LT, Ding JY, Zhang CM, Jia JW, Weng HB, Liu WX, Zhang DB (2005) Estimating the copy number of transgenes in transformed rice by real-time quantitative PCR. Plant Cell Rep 23:759–763
Zimdahl RL (2007) Fundamental of weed science, 3rd edn. Academic Press, New York, p 325
Acknowledgements
This research was supported by a grant from the Scientific and Technological Council of Turkey (Tübitak), Project No. 115O022. Authors thank Ms. Nurefşan Cırık for her help in transformation experiments. We also thank Dr. Halil Toktay for providing access to microscopes.
Author information
Authors and Affiliations
Contributions
AB designed the study, constructed recombinant vector, optimized transformation protocol for potato cultivars and supervised overall activities of the project. TH and IR did genetic transformation experiments, UD and MEÇ made significant contribution to molecular and application assays of transgenic plants.
Corresponding author
Ethics declarations
Conflict of interest
Authors declare no conflict of interest.
Additional information
Communicated by Manoj Prasad.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Bakhsh, A., Hussain, T., Rahamkulov, I. et al. Transgenic potato lines expressing CP4-EPSP synthase exhibit resistance against glyphosate. Plant Cell Tiss Organ Cult 140, 23–34 (2020). https://doi.org/10.1007/s11240-019-01708-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11240-019-01708-1