Abstract
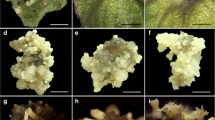
Carnation is an important cut flower with industrial and medicinal applications. To establish an efficient protocol without somaclonal variation for micropropagation of Dianthus caryophyllus, direct and indirect somatic embryogenesis (DSE and ISE) were investigated under six different light spectra (white, red, green, blue, red + blue and far red + red) and four combinations of different plant growth regulators (PGRs) never tested so far for carnation. The best results were achieved with 2,4-dichlorophenoxyacetic acid (2,4-D) + N-(2-chloro-4-pyridyl)-N′-phenylurea (4-CPPU) for ISE and picloram + 4-CPPU or naphthoxyacetic acid (NOA) + 6-benzylaminopurine (BAP) for DSE. The DSE method was faster (3 weeks compared to 8 weeks) and easier (no subculturing compared to two rounds of subculture with ISE methods) but the percentage of somatic embryos in the ISE method was higher compared to the DSE method. Our results showed that the highest DSE, formation of embryogenic callus, embryo maturation (generation of globular, heart and torpedo shapes) and ISE rate was observed in carnation explants exposed to blue light (450–495 nm). In contrast, green (495–570 nm), red (610–700) and far red (710–730 nm) lights caused negative effects on embryogenesis compared to white light controls (380–750 nm). For the first time, genetic stability of regenerated carnation plants was estimated using inter-simple sequence repeat (ISSR) markers. The amplified products showed 75 distinct and scorable bands, and regenerants [plants obtained by primary (PSE) and secondary SE (SSE)] were completely identical to the mother plant. Similarly, flow cytometric analysis confirmed that somatic embryo-derived plants had on average 1.53 pg nuclear DNA (2C), and all plants maintained their ploidy. In conclusion, obtained embryos under blue light were big in size and torpedo-shaped and their germination was highest compared to other light spectra. Moreover, blue light was effective for direct and indirect somatic embryogenesis in carnation without induction of somaclonal variation.
Key message
An effective protocol through application of phytohormones is introduced. Blue light can be used to improve in vitro propagation of carnation by somatic embryogenesis. Genetic stability of regenerated carnation plants was confirmed using inter-simple sequence repeat (ISSR) markers.





Similar content being viewed by others
References
Agulló-Antón MÁ, Olmos E, Pérez-Pérez JM, Acosta M (2013) Evaluation of ploidy level and endoreduplication in carnation (Dianthus spp.). Plant Sci 201:1–11
Ali M, Mujib A, Tonk D, Zafa N (2016) Plant regeneration through somatic embryogenesis and genome size analysis of Coriandrum sativum L. Protoplasma 254:343–352
Bennett MD, Bhandol P, Leitch IJ (2000) Nuclear DNA amounts in angiosperms and their modern uses—807 new estimates. Ann Bot 86(4):859–909
Bhattacharya C, Dam A, Karmakar J, Bandyopadhyay TK (2016) Direct somatic embryogenesis and genetic homogeneity assessment of regenerated plants of Anthurium andraeanum Linden cv. Fantasia. In Vitro Cell Dev Biol Plant 52:512–519
Bornet B, Branchard M (2001) Non anchored inter simple sequence repeat (ISSR) markers: reproducible and specific tools for genome fingerprinting. Plant Mol Biol Rep 19:209–215
Boufis N, Khelifi-Slaoui M, Djillali Z, Zaoui D, Morsli A, Bernards M, Khelifi L (2014) Effects of growth regulators and types of culture media on somatic embryogenesis in date palm (Phoenix dactylifera L. cv. Degla Beida). Sci Hortic 172:135–142
Brito G, Loureiro J, Lopes T, Rodriguez E, Santos C (2008) Genetic characterization of olive trees from Madeira Archipelago using flow cytometry and microsatellite markers. Genet Resour Crop Evol 55:657–664
Burch LR, Horgan R (1989) The purification of cytokinin oxidase from Zea mays kernels. Phytochemistry 28:1313–1319
Burich GA, Mercun P, Benedtti L, Giovannini A (1996) Transformation method applicable to ornamental plant. Plant Tissu Cuit Biotech 12:94–104
Carloni E, Ribotta A, Colomba EL, Griffa S, Quiroga M, Tommasino E, Grunberg K (2014) Somatic embryogenesis from in vitro anther culture of apomictic buffel grass genotypes and analysis of regenerated plants using flow cytometry. Plant Cell Tissue Organ Cult 117(3):311–322
Carra A, Sajeva M, Abbate L, Siragusa M, Pathirana R, Carimi F (2016) Factors affecting somatic embryogenesis in eight Italian grapevine cultivars and the genetic stability of embryo-derived regenerants as assessed by molecular markers. Sci Hortic 204:123–127
Chen JR, Wu L, Hu BW, Yi X, Liu R, Deng ZN, Xiong XY (2014) The influence of plant growth regulators and light quality on somatic embryogenesis in China rose (Rosa chinensis Jacq.). J Plant Growth Regul 33:295–304
Correia S, Cunha AE, Salgueiro L, Canhoto JM (2012) Somatic embryogenesis in Tamarillo (Cyphomandra betacea): approaches to increase efficiency of embryo formation and plant development. Plant Cell Tissue Organ Cult 109:143–152
Cowan MM (1999) Plant products as antimicrobial agents. Clin Microbiol Rev 12:564–582
D’Onofrio C, Morini C, Bellocchi G (1998) Effect of light quality on somatic embryogenesis of quince leaves. Plant Cell Tissue Organ Cult 53:91–98
Doležel J, Bartos J (2005) Plant DNA flow cytometry and estimation of nuclear genome size. Ann Bot 95:99–110
Doležel J, Sgorbati S, Lucretti, S (1992) Comparison of three DNA fluorochromes for flow cytometric estimation of nuclear DNA content in plants. Physiol Plant 85(4):625–631
Dolezel J, Greilhuber J, Suda J (2007) Estimation of nuclear DNA content in plants using flow cytometry. Nat Protoc 2:2233–2244
Fiore MC, Carimi F, Carra A, Sunseri F (2012) Efficient plant regeneration via somatic embryogenesis in bulbing fennel using immature flowers explants. In Vitro Cell Dev Biol Plant 48:440–445
Frey L, Saranga Y, Janick J (1992) Somatic embryogenesis in carnation. Hort Sci 27:63–65
Ghimire BK, Yu CY, Chung IM (2012) Direct shoot organogenesis and assessment of genetic stability in regenerants of Solanum aculeatissimum Jacq. Plant Cell Tissue Organ Cult 108:455–464
Gow WP, Chen JT, Chang WC (2009) Effects of genotype, light regime, explant position and orientation on direct somatic embryogenesis from leaf explants of Phalaenopsis orchids. Acta Physiol Plant 31:363–369
Hasebe M, Iwatsuki K (1990) Adiantum capillus-veneris chloroplast DNA clone bank: as useful heterologous probes in the systematics of the leptosporangiate ferns. Am Fern J 80:20–25
Huan VT, Tanaka M (2004) Callus induction from protocorm-like body segments and plant regeneration in Cymbidium (Orchidaceae). J Hortic Sci Biotechnol 79:406–410
Iantcheva A, Vlahova M, Atanassova B, Atanassov A (2005) Plant regeneration via direct organogenesis and somatic embryogenesis of two new Bulgarian spray carnation cultivars. Biotechnol Biotechnol Equip 19:15–19
Jha TB, Mukherjee P, Datta MM (2007) Somatic embryogenesis in Jatropha curcas Linn., an important biofuel plant. Plant Biotech Rep 1:135–140
Jin S, Mushke R, Zhu H, Tu L, Lin Z, Zhang Y, Zhang X (2008) Detection of somaclonal variation of cotton (Gossypium hirsutum) using cytogenetics, flow cytometry and molecular markers. Plant Cell Rep 27(8):1303–1316
Ju HJ, Jeyakumar J, Kamaraj M, Praveen N, Chung IM, Kim SH, Thiruvengadam M (2014) High frequency somatic embryogenesis and plant regeneration from hypocotyl and leaf explants of gherkin (Cucumis anguria L.). Sci Hortic 169:161–168
Kaldenhoff R, Henningsen U, Richter G (1994) Gene activation in suspension-cultured cells of Arabidopsis thaliana during blue-light-dependent plantlet regeneration. Planta 195:182–187
Karami O (2008) Induction of embryogenic callus and plant regeneration in Carnation (Dianthus caryophyllus L.). J Biol Sci 8:68–72
Karami O, Kordestani GK (2007) Proliferation, shoot organogenesis and somatic embryogenesis in embryogenic callus of carnation. J Fruit Ornamental Plant Res 15:167–175
Karami O, Deljou A, Esna-Ashari M, Ostad-Ahmadi P (2006) Effect of sucrose concentrations on somatic embryogenesis in carnation (Dianthus caryophyllus L.). Sci Hortic 110:340–344
Karami O, Deljou A, Kordestani GK (2008) Secondary somatic embryogenesis of carnation (Dianthus caryophyllus L.). Plant Cell Tissue Organ Cult 92:273–280
Kintzios SE, Taravira N (1997) Effect of genotype and light intensity on somatic embryogenesis and plant regeneration in melon (Cucumis melo L.). Plant Breed 116:359–362
Konieczny R, Sliwinska E, Pilarska M, Tuleja M (2012) Morphohistological and flow cytometric analyses of somatic embryogenesis in Trifolium nigrescens Viv. Plant Cell Tissue Organ Cult 109:131–141
Kumar S, Kumari R, Baheti T, Thakur M, Ghani M (2016) Plant regeneration from axillary bud, callus and somatic embryo in carnation (Dianthus caryophyllus) and assessment of genetic fidelity using RAPD-PCR analysis. Indian J Agric Sci 86:1482–1488
LoSchiavo F, Pitto L, Giuliano G, Torti G, Nuti-Ronchi V, Marazziti D, Terzi M (1989) DNA methylation of embryogenic carrot cell cultures and its variations as caused by mutation, differentiation, hormones and hypomethylating drugs. Theor Appl Genet 77:325–331
Martin KP, Madassery J (2005) Direct and indirect somatic embryogenesis on cotyledon explants of Quassia amara L., an antileukaemic drug plant. In Vitro Cell Dev Biol 41:54–57
Mengxi L, Zhigang X, Yang Y, Yijie F (2011) Effects of different spectral lights on Oncidium PLBs induction, proliferation, and plant regeneration. Plant Cell Tissue Organ Cult 106:1–10
Mohammed MJ, Al-Bayati FA (2009) Isolation and identification of antibacterial compounds from Thymus kotschyanus aerial parts and Dianthus caryophyllus flower buds. Phytomedicine 16:632–637
Morais-Lino LS, Santos-Serejo JA, Amorim EP, Santana JRF, Pasqual M, Silva SO (2016) Somatic embryogenesis, cell suspension, and genetic stability of banana cultivars. In Vitro Cell Dev Biol 52:99–106
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15:473–497
Naing AH, Min JS, Park KI, Chung MY, Lim SH, Lim KB, Kim CK (2013) Primary and secondary somatic embryogenesis in Chrysanthemum (Chrysanthemum morifolium) cv. ‘Baeksun’ and assessment of ploidy stability of somatic embryogenesis process by flow cytometry. Acta Physiol Plant 35:2965–2974
Nakano M, Mii M (1992) Protoplast culture and plant regeneration of several species in the genus Dianthus. Plant Cell Rep 11:225–228
Navarro-García N, Morte A, Pérez-Tornero O (2016) In vitro adventitious organogenesis and histological characterization from mature nodal explants of Citrus limon. In Vitro Cell Dev Biol 52(2):161–173
Neelakandan AK, Wang K (2012) Recent progress in the understanding of tissue culture-induced genome level changes in plants and potential applications. Plant Cell Rep 31:597–620
Nimura M, Kato J, Horaguchi H, Mii M, Sakai K, Katoh T (2006) Induction of fertile amphidiploids by artificial chromosome-doubling in interspecific hybrid between Dianthus caryophyllus L. and D. japonicus Thunb. Breed Sci 56(3):303–310
Pareek A, Kothari SL (2003) Direct somatic embryogenesis and plant regeneration from leaf cultures of ornamental species of Dianthus. Sci Hortic 98(4):449–459
Park YS, Lelu-Walter MA, Harvengt L, Trontin JF, MacEacheron I, Klimaszewska K, Bonga JM (2006) Initiation of somatic embryogenesis in Pinus banksiana, P. strobus, P. pinaster, and P. sylvestris at three laboratories in Canada and France. Plant Cell Tissue Organ Cult 86:87–101
Paul S, Dam A, Bhattacharyya A, Bandyopadhyay TK (2011) An efficient regeneration system via direct and indirect somatic embryogenesis for the medicinal tree Murraya koenigii. Plant Cell Tissue Organ Cult 105:271–283
Pinto DLP, Barros BA, Viccini LF, Campos JMS, Silva ML, Otoni WC (2010) Ploidy stability of somatic embryogenesis-derived Passiflora cincinnata Mast. plants as assessed by flow cytometry. Plant Cell Tissue Organ Cult 103:71–79
Prakash MG, Gurumurthi K (2010) Effects of type of explant and age, plant growth regulators and medium strength on somatic embryogenesis and plant regeneration in Eucalyptus camaldulensis. Plant Cell Tissue Organ Cult 100(1):13
Prange ANS, Serek M, Bartsch M, Winkelmann T (2010) Efficient and stable regeneration from protoplasts of Cyclamen coum Miller via somatic embryogenesis. Plant Cell Tissue Organ Cult 101:171–182
Rai MK, Phulwaria M, Harish M, Gupta AK, Shekhawat NS, Jaiswal U (2012) Genetic homogeneity of guava plants derived from somatic embryogenesis using SSR and ISSR markers. Plant Cell Tissue Organ Cult 111:259–264
Raji MR, Lotfi M, Tohidfar M, Zahedi B, Carra A, Abbate L, Carimi F (2018) Somatic embryogenesis of muskmelon (Cucumis melo L.) and genetic stability assessment of regenerants using flow cytometry and ISSR markers. Protoplasma 255:873–883
Ramawat KG, Mathur M (2007) Factors affecting the production of secondary metabolites. Biotechnology: secondary metabolites. Plants Microbes 46:59–102
Ray T, Dutta I, Saha P, Das S, Roy SC (2006) Genetic stability of three economically important micropropagated banana (Musa spp.) cultivars of lower Indo-Gangetic plains as assessed by RAPD and ISSR markers. Plant Cell Tissue Organ Cult 85:11–21
Rodríguez-Sahagún A, Acevedo-Hernández G, Rodríguez-Domínguez J, Rodríguez-Garay B, Cervantes-Martínez J, Castellanos-Hernández O (2011) Effect of light quality and culture medium on somatic embryogenesis of Agave tequilana Weber var Azul. Plant Cell Tissue Organ Cult 104:271–275
Sahijram L, Bahadur B (2015) Plant biology and biotechnology. In: Bahadur B, Rajam MV, Sahijram L, Krishnamurthy KV (eds) Somatic embryogenesis. Springer, India, pp 315–327
Seo J, Weon Kim S, Ran Min S (2007) High frequency somatic embryogenesis and plant regeneration in root explant cultures of carnation. Plant Biotech Rep 1:67–70
Singh R, Kashyap SP, Kumari N, Singh M (2016) Regeneration of soapnut tree through somatic embryogenesis and assessment of genetic fidelity through ISSR and RAPD markers. Physiol Mol Biol Plants 22:381–389
Siragusa M, Carra A, Salvia L, Puglia AM, De Pasquale F, Carimi F (2007) Genetic instability in calamondin (Citrus madurensis Lour.) plants derived from somatic embryogenesis induced by diphenylurea derivatives. Plant Cell Rep 26:1289–1296
Szopa A, Ekiert H (2016) The importance of applied light quality on the production of lignans and phenolic acids in Schisandra chinensis (Turcz.) Baill cultures in vitro. Plant Cell Tissue Organ Cult 127:115–121
Torne JM, Moysset L, Santos M, Simon E (2001) Effects of light quality on somatic embryogenesis in Araujia sericifera. Physiol Plant 111:405–411
Victor JMR, Murthy BNS, Murch SJ, Saxena PK (1999) Role of endogenous purine metabolism in thidiazuron induced somatic embryogenesis of peanut (Arachis hypogaea L.). Plant Growth Regul 28:41–47
Viehmannova I, Cepkova PH, Vitamvas J, Streblova P, Kisilova J (2016) Micropropagation of a giant ornamental bromeliad Puya berteroniana through adventitious shoots and assessment of their genetic stability through ISSR primers and flow cytometry. Plant Cell Tissue Organ Cult 125(2):293–302
Virkrant A, Rashid A (2001) Direct as well as indirect somatic embryogenesis from immature (unemerged) inflorescence of a minor millet Paspalum scrobiculatum L. Euphytica 120:167–172
Weis JS, Jaffe MJ (1969) Photoenhancement by blue light of organogenesis in tobacco pith cultures. Physiol Plant 22:171–176
Yagi M, Kosugi S, Hirakawa H, Ohmiya A, Tanase K, Harada T, Tabata S (2013) Sequence analysis of the genome of carnation (Dianthus caryophyllus L.). DNA Res 21:231–241
Yantcheva A, Vlahova M, Atanassov A (1998) Direct somatic embryogenesis and plant regeneration of carnation (Dianthus caryophyllus L.). Plant Cell Rep 18:148–153
Acknowledgements
LED lights were provided by Iran grow light company (http://www.Irangrowlight.ir). The authors wish to thank Dr Christian Gehl, Faculty of Natural Sciences, Institute of Horticultural Production Systems, Floriculture, Leibniz University Hannover, Germany, for his thoughtful and inspirational comments. We would like to thank Iran National Science Foundation (INSF) (grant number 96006991) and University of Tehran for their supports.
Author information
Authors and Affiliations
Contributions
MF, MA and SA performed most in vitro culture experiments and together with SD, MZ, and MF contributed to in vitro initiation experiments; MF and MZ performed the genetic analyses by ISSR markers; MA conceived and designed the experiments and together with MF, SA, EL, MS and MZ contributed to data interpretation. MF, EL, and MS contributed mostly to manuscript elaboration and other authors contributed to its revision.
Corresponding authors
Additional information
Communicated by Sergey V Dolgov.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Aalifar, M., Arab, M., Aliniaeifard, S. et al. Embryogenesis efficiency and genetic stability of Dianthus caryophyllus embryos in response to different light spectra and plant growth regulators. Plant Cell Tiss Organ Cult 139, 479–492 (2019). https://doi.org/10.1007/s11240-019-01684-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11240-019-01684-6