Abstract
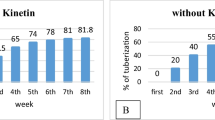
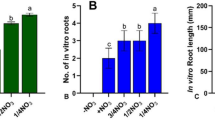
Gloriosa superba L. tubers are a rich source of commercially important colchicine and due to overexploitation, the species has become vulnerable. In the present investigation, in vitro tuber productions were carried out for its propagation and conservation. The in vitro and field-grown tubers were assessed for their colchicine content and antimicrobial activities. Maximum callusing was obtained when the medium was supplemented with 2,4-dichlorophenoxyacetic acid (2,4-D) and 6-furfurylaminopurine (kinetin). Among different auxins tested for in vitro tuberous root production, IAA (1.5 mg/L) induced 78.2% tuberous root per callus. In vitro micro-tuber raised in media supplemented with 1.5 mg/L thidiazuron (TDZ) recorded the highest response (72.9%) with 28.4 tubers per explant. Sucrose (6%) with TDZ (1.5 mg/L) produced significantly more micro-tubers per callus. Elicitor treatment with AlCl3 at 125 µM and 150 µM resulted in a significant increase in the micro-tuber and tuberous root production respectively. N6-(2-Isopentenyl) adenine (2ip) (1.0 mg/L) induced the highest frequency of in vitro micro-tuber sprouting and tuber formation compared to 6-benzylaminopurine (BAP) and 1-naphthalene acetic acid (NAA). The elicitor-treatments with AlCl3 significantly increased the colchicine content of in vitro tuberous root and tubers than that of the field grown tubers. The anti-microbial activity of in vitro raised tubers, tuberous roots and AlCl3 treated samples were significantly higher compared to the field grown samples. An optimized tissue culture system for mass propagation of G. superba with conservation aspects and the production of high-value colchicine is presented here, which can be used in various medicinal systems.
Key message
Manipulation of PGR’S significantly enhanced in vitro tuberization from noncorm bud explants. Elicitor treatment with AlCl3 enhanced the production of colchicine. HPLC analysis revealed significantly higher colchicine content in in vitro raised plants compared to field grown tubers. The study will help in mass propagation, conservation, and commercialization of Gloriosa superba L. for large scale production of colchicine.




Similar content being viewed by others
References
Anandhi S, Rajamani K (2012) In vitro tuberization of glory lily (Gloriosa superba L.). J Hortic For 4:81–84
Anandhi S, Rajamani K, Jawaharlal M (2013) Propagation studies in Gloriosa superba. Med Aromat Plant Res J 1(1):1–4
Arumugam A, Gopinath K (2012) In vitro micropropagation using corm bud explants: an endangered medicinal plant of Gloriosa superba L. Asian J Biotechnol 4:120–128
Basak UC, Dash D, Mahapatra AK (2012) Estimation of colchicine in tubers of Gloriosa superba L. originated from different agroclimatic zones of Odisha, India. Int J Pharmacog Phytochem Res 4:157–161
Bhakuni DS, Jain S (1995) In: Chadha KL, Gupta R (eds) Advances in horticulture, vol 11. Malhotra Publishing House, Delhi, pp 98–99
Budhiraja A, Nepali K, Sapra S, Gupta S, Kumar S, Dhar KL (2013) Bioactive metabolites from an endophytic fungus of Aspergillus species isolated from seeds of Gloriosa superba Linn. Med Chem Res 22:323–329
Chen H, Chen F (2000) Effect of yeast elicitor on the secondary metabolism of Ti transformed Salvia miltiorrhiza cell suspension cultures. Plant Cell Rep 19:710–717
Chen H, Chen F, Chiu Francis CK, Lo Cindy MY (2001) The effect of yeast elicitor on the growth and secondary metabolism of hairy root cultures of Salvia miltiorrhiza. Enzyme Micro Technol 28:100–105
Custers JBM, Bergervoet JHW (1994) Micropropagation of Gloriosa: towards a practical protocol. Sci Hortic 57(4):323–334
Farooqi AA, Kumaraswamy BK, Bojappa KN, Pusalkar VR, Gupta R (1993) Plantations of the clinically important Gloriosa superba. Indian Hortic 37:26–29
Finnie JF, Van Staden J (1989) In vitro propagation of Sandersonia and Gloriosa. Plant Cell Tissue Organ Cult 19:151–158
Ghosh B, Mukherjee S, Jha TB, Jha S (2002) Enhanced colchicine production in root cultures of Gloriosa superba by direct and indirect precursors of the biosynthetic pathway. Biotechnol Lett 24:231–234
Ghosh S, Ghosh B, Jha S (2006) Aluminium chloride enhances colchicine production in root cultures of Gloriosa superba. Biotechnol Lett 28:497–503
Ghosh S, Ghosh B, Jha S (2007) In vitro tuberisation of Gloriosa superba L. on basal medium. Sci Hortic 114:220–223
Gontier E, Sangwan BS, Barbotin JN (1994) Effects of calcium, alginate, and calcium alginate immobilization on growth and tropane alkaloid levels of a stable suspension cell line of Daturainnoxia Mill. Plant Cell Rep 13:533–536
Gunawan LW, Sutanto K, Lay SF (1990) In vitro shoot multiplication and plantlet formation of Gloriosa superba Linn. In: Abstracts of 7th international congress on plant tissue and cell culture. Amsterdam 24-29 IAPTC 103
Gupta BK (1999) Production of colchicine from G. superba tubers in cultivation and utilization of medicinal plants. CSIR J 1999:270–278
Hamel F, Breton C, Houde M (1998) Isolation and characterization of wheat aluminum-regulated genes: possible involvement of aluminum as a pathogenesis response elicitor. Planta 205:531–538
Hannapel DJ, Sharma P, Lin T, Banerjee AK (2017) The multiple signals that control tuber formation. Plant Physiol 174:845–856
Jadhav SY, Hegde BA (2001) Somatic embryogenesis and plant regeneration in Gloriosa L. Indian J Exp Biol 39:943–946
Johnson WC, William OW (2002) Warfarin toxicity. J Vasc Surg 35:413–421
Khan H, Ali Khan M, Mahmood T, Choudhary MI (2008) Antimicrobial activities of Gloriosa superba Linn (Colchicaceae) extracts. J Enzyme Inhib Med Chem 23:855–859
Khandel AK, Khan S, Ganguly S, Bajaj A (2011) In vitro shoot initiation from apical shoot buds & meristems of Gloriosa superba L.—an endangered medicinal herb of high commercial value. Research 3:36–45
Kirby WM, Yoshihara GM, Sundsted KS, Warren JH (1956) Clinical usefulness of a single disc method for antibiotic sensitivity testing. Antibiot Annu 1956–1957:892–897
Krause J (1986) Production of Gloriosa tubers from seeds. Acta Hortic 177:353–360
Kumar CN, Jadhav SK, Tiwari KL, Afaque Q (2015) In vitro Tuberization and colchicine content analysis of Gloriosa superba L. Biotechnology 14:142–147
Llina´s RR (1999) The squid giant synapse: a model for chemical transmission. Oxford University Press, London
Mahajan R, Kapoor N, Billowria P (2016) Callus proliferation and in vitro organogenesis of Gloriosa superba: an endangered medicinal plant. Ann Plant Sci 5:1466–1471
Mukherjee S, Ghosh B, Jha S (2000) Enhanced forskolin production in genetically transformed cultures of Coleus forskohlii by casein hydrolysate and studies on growth and organization. Biotechnol Lett 22:133–136
Murashige T, Skoog F (1962) A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol Plant 15:473–497
Naik PM, Al-Khayri J (2016) Abiotic and biotic elicitors—role in secondary metabolites production through in vitro culture of medicinal plants. In: Shanker AK, Shanker C (eds) Abiotic and biotic stress in plants—recent advances and future perspectives. INTECH, London, pp 247–277
Nayak S (2000) In vitro multiplication and microrhizome induction in Curcuma aromatica Salisb. Plant Growth Regul 32:41–47
Nikhila GS, Sangeetha G, Nair AG, Pradeesh S, Swapna TS (2014) High frequency embryogenesis and organogenesis in Gloriosa superba L.—a plant in need of conservation. J Aquat Biol Fish 2:398–402
Pandurangan B, Philomina D (2010) Effect of nutritional factors and precursors on formation of colchicine in Gloriosa superba in vitro. Res Biotechnol 1:29–37
Pitta-Alvarez SI, Spollansky TC, Giulietti AM (2000) Scopolamine and hyoscyamine production by hairy root cultures of Brugmansia candida: influence of calcium chloride, hemicellulase and theophylline. Biotechnol Lett 22:1653–1656
Prematilake DP, Mendis MH (1999) Micro-tuber of potato (Solanum tuuberosum L.) in vitro conservation and tissue culture. J Nat Sci Found Sri Lanka 27:17–28
Rai VR (2002) Rapid clonal propagation of Nothapodytes foetida (Wight) sleumer—a threatened medicinal tree. In Vitro Cell Dev Biol - Plant 38:347–351
Rao KB, Ramesh NP, Lakshmana Swamy P, Muralinath E (2014) In-vitro investigation on antimicrobial activity of Gloriosa superba Linn tubers against major food borne pathogens. Am J Drug Deliv Ther 1:009–020
Rishi A (2011) In vitro callus induction and regeneration of healthy plants of Gloriosa superba Linn. Indian J Fundam Appl Life Sci 1:64–65
Saikia M, Shrivastava K, Singh SS (2013) Effect of culture media and growth hormones on callus induction in Aquilaria malaccensis Lam., a medicinally and commercially. Asian J Biol Sci 6:96–105
Satish L, Rathinapriya P, Rency AS, Ceasar SA, Pandian S, Rameshkumar R, Ramesh M (2016) Somatic embryogenesis and regeneration using Gracilaria edulis and Padina boergesenii seaweed liquid extracts and genetic fidelity in finger millet (Eleusine coracana). J Appl Phycol 28:2083–2098
Shimasaki K, Sakuma K, Nishimura Y (2007) Tuber formation of Gloriosa superba using stem sections of branches under cultivation. In: III international symposium on acclimatization and establishment of micropropagated plants, vol 812, pp 245–250
Siva G, Sivakumar S, Prem Kumar G, Vigneswaran M, Vinoth S, Selvan AM, Senthil Kumar T, Jayabalan N (2015) Optimization of elicitation condition with jasmonic acid, characterization and antimicrobial activity of psoralen from direct regenerated plants of Psoralea corylifolia L. Biocatal Agric Biotechnol 4:624–631
Sivakumar G (2002) Gloriosa superba L. a very useful medicinal plant. In VK Singh, JN Govil, S Hashmi, G Singh, eds, Recent Progress in Medicinal Plants, Vol. 7, Ethnomedicine and Pharmacognosy, Part II. Series Sci Tech Pub Texas USA 465:82465-482
Sivakumar G, Krishnamurthy KV (2004) In vitro organogenetic responses of Gloriosa superba. Russian J Plant Physiol 51(5):713–721
Sivakumar G, Krishnamurthi KV, Rajendran TD (2003a) In vitro corm production in Gloriosa superba L., an ayurvedic medicinal plant. J Hortic Sci Biotechnol 78:450–453
Sivakumar G, Krishnamurthy KV, Rajendran TD (2003b) Embryoidogenesis and plant regeneration from leaf tissue of Gloriosa superba. Planta Med 69:479–481
Sivakumar G, Krishnamurthy KV, Hahn EJ, Paek KY (2004) Enhanced in vitro production of colchicine in Gloriosa superba L. an emerging industrial medicinal crop in South India. J Hortic Sci Biotech 79:602–605
Spollansky TC, Pitta-Alvarez SI, Giulietti AM (2000) Effect of jasmonic acid and aluminium on production of tropane alkaloids in hairy root cultures of Brugmansia candida. Electron J Biotechnol 3:72–75
Szabo E, Thelen A, Peterson M (1999) Fungal elicitor preparations and methyl jasmonate enhance rosmarinic acid accumulation in suspension cultures of Coleus blumei. Plant Cell Rep 18:485–489
Thakur RS, Potesllova H, Santavy F (1975) Substances from plants of the subfamily Wurmbaeoideae and their derivatives. Part LXXIX. Alkaloids of the plant Gloriosa superba L. Planta Med 28:201–209
Von der Weid I, Alviano DS, Santos ALS, Soares RMA, Alviano CS, Seldin L (2003) Antimicrobial activity of Paenibacillus peoriae strain NRRL BD-62 against a broad spectrum of phytopathogenic bacteria and fungi. J Appl Microbiol 95:1143–1151
Yadav K, Aggarwal A, Singh N (2012) Actions for ex situ conservation of Gloriosa superba L.—an endangered ornamental cum medicinal plant. J Crop Sci Biotechnol 15:297–303
Yadav K, Aggarwal A, Singh N (2013) Arbuscula rmycorrhizal fungi (AMF) induced acclimatization, growth enhancement and colchicine content of micropropagated Gloriosa superba L. plantlets. Ind Crop Prod 45:88–93
Yue W, Ming QL, Lin B, Rahman K, Zheng CJ, Han T, Qin LP (2016) Medicinal plant cell suspension cultures: pharmaceutical applications and high-yielding strategies for the desired secondary metabolites. Crit Rev Biotechnol 36:215–232
Acknowledgements
Dr. S. Sivakumar would like to acknowledge the Department of Plant Science, Bharathidasan University, Tiruchirappalli for providing HPLC facility (UGC-SAP) for this research. Mr. S. Sathish acknowledges ICMR, New Delhi (No. 3/1/2/102/2018-Nut.) for fellowship support.
Author information
Authors and Affiliations
Contributions
SS designed and executed all the experiments. SS prepared the manuscript. GS, SV, GPK, PG, and MV contributed substantially in experimental analysis and discussion. TSK, RS, and NJ mobilized funds and critically evaluated the manuscript.
Corresponding author
Additional information
Communicated by Wagner Campos Otoni.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Subiramani, S., Sundararajan, S., Govindarajan, S. et al. Optimized in vitro micro-tuber production for colchicine biosynthesis in Gloriosa superba L. and its anti-microbial activity against Candida albicans. Plant Cell Tiss Organ Cult 139, 177–190 (2019). https://doi.org/10.1007/s11240-019-01675-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11240-019-01675-7