Abstract
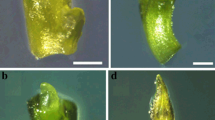
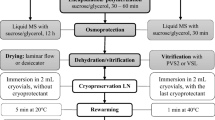
Encapsulation-dehydration, encapsulation-vitrification, and vitrification were tested for cryopreservation of Lotus tenuis (Fagaceae) adventitious buds clusters (ABCs) obtained by a direct regeneration system from leaves cultures. Among them, the PVS3-based vitrification procedure was found to be useful for survival and regrowth of the preserved explants. For vitrification, the ABCs were dehydrated in a solution containing 2 M glycerol + 0.4 M sucrose for 25 min at room temperature, submerged in PVS3 solution for 1 h at 0 °C, then immersed in liquid nitrogen for 48 h and rapidly rewarmed. Afterword, the explants were unloaded in MS liquid medium with 1.2 M sucrose for 30 min. The washed tissues were dried superficially on filter paper and cultured in semisolid hormone-free MS medium containing 0.1 M sucrose. All cultures were maintained at 25 °C in the dark for 10 days and transferred to the light conditions. With this procedure, 79 ± 5.3% survival and more than 80% of the plantlets displaying a phenotype similar to the non-treated control after acclimatization. The data settled from ISSR showed no genetic dissimilarities between in vitro regenerants derived from cryopreserved tissues and the non-treated plants. Thus, our results indicate that the use of vitrification-based PVS3 solution offers a simple, accurate, and appropriate procedure for the cryopreservation of L. tenuis adventitious buds.






Similar content being viewed by others
References
Balzarini M, Di Rienzo J (2013) Info-Gen: software para análisis estadístico de datos genéticos. Facultad de Ciencia Agropecuarias, Universidad Nacional de Córdoba, Córdoba. http://www.info-gen.com.ar
Brugnoli E, Urbani M, Quarin C, Martínez E, Acuña C (2013) Diversity in diploid, tetraploid, and mixed diploid–tetraploid populations of Paspalum simplex. Crop Sci 53:1509–1516
Dumet D, Benson E (2000) The use of physical and biochemical studies to elucidate and reduce cryopreservation-induced damage in hydrated/desiccated plant germplasm. In: Engelmann F, Takagi H (eds) Cryopreservation of tropical plant germplasm. Current research progress and application. IPGRI, JIRCAS International Agriculture Roma, Rome, pp 43–56
Escaray FJ, Menéndez AB, Gárriz A, Ruiz OA (2012) Ecological and agronomic importance of the plant genus Lotus. Its application in grassland sustainability and the amelioration of constrained and contaminated soils. Plant Sci 182:121–133
Espasandin F, Collavino M, Luna C, Tarragó J, Paz R, Ruiz OA, Mroginski L, Sansberro P (2010) Agrobacterium tumefaciens-mediated transformation of Lotus tenuis Mill. and regeneration of transgenic lines. Plant Cell Tissue Organ Cult 102:181–189
Espasandin F, Maiale S, Calzadilla P, Ruiz O, Sansberro P (2014) Transcriptional regulation of 9-cis-epoxycarotenoid dioxygenase (NCED) gene by putrescine accumulation positively modulates ABA synthesis and drought tolerance in Lotus tenuis plants. Plant Physiol Biochem 76:29–35
Espasandin F, Calzadilla P, Maiale S, Ruiz O, Sansberro P (2017) Overexpression of the arginine decarboxylase gene improves tolerance to salt stress in Lotus tenuis plants. J Plant Growth Regul 37:156–165
Fahy GM (1986) The relevance of cryoprotectant toxicity to cryobiology. Cryobiology 23:1–13
Ferraro G, Filip R, del Pero MA, Basualdo N, Mendoza R, García I (2010) Flavonoids of Lotus tenuis (Waldst. & Kit.) as markers of populations growing in soils of different saline and hydrologic conditions. J Braz Chem Soc 21:1739–1745
Ferreira J, Pedrosa-Harand A (2014) Lotus cytogenetics. In: Tabata S, Stougaard J (eds) The Lotus japonicus genome. Springer, Berlin, pp 9–20
Ferreira Nogueira G, Pasqual M, Scherwinski–Pereira JE (2013) Survival of sugarcane shoot tips after cryopreservation by droplet-vitrification. Pesqui Agropecu Bras 48:1524–1527
González-Arnao MT, Engelmann F (2013) Consideraciones teóricas y prácticas para la crioconservación de germoplasma vegetal. In: Crioconservación de plantas en América Latina y el Caribe. Instituto Interamericano de Cooperación para la Agricultura, San José, pp 37–53
Hirai D, Sakai A (1999) Cryopreservation of in vitro-grown axillary shoot-tip meristems of mint (Mentha spicata L.) by encapsulation vitrification. Plant Cell Rep 9:150–155
Kaczmarczyk A, Funnekotter B, Menon A, Phang PY, Al-Hanbali A, Bunn E, Mancera RL (2012) Current issues in plant cryopreservation. In: Katkov I (ed) Current frontiers in cryobiology. InTech, Shanghai, pp 418–438
Karlsson JOM, Toner M (1996) Long-term storage of tissues by cryopreservation: critical issues. Biomaterials 17:243–256
Kim HH, Lee YG, Shin DJ, Ko HC, Gwag JG, Cho EG, Engelmann F (2009) Development of alternative plant vitrification solutions to be used in droplet-vitrification procedures. Acta Hortic 908:181–186
Makowska Z, Keller J, Engelmann F (1999) Cryopreservation of apices isolated from garlic (Allium sativum L.) bulbils and cloves. CryoLetters 20:175–182
Markovic Z, Chatelet P, Sylvestre I, Kontić JK, Engelmann F (2013) Cryopreservation of grapevine (Vitis vinifera L.) in vitro shoot tips. Cent Eur J Biol 8:993–1000
Matsumoto T (2017) Cryopreservation of plant genetic resources: conventional and new methods. Rev Agric Sci 5:13–20
Matsumoto T, Sakai A, Takahashi C, Yamada K (1995) Cryopreservation in vitro grown apical meristems of wasabi (Wasabi japonica) by encapsulation vitrification method. CryoLetters 16:189–206
Montes L (1988) Lotus tenuis. Rev Argent Prod Anim 8:367–376
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue culture. Physiol Plant 15:473–497
Nishizawa S, Sakai A, Amano Y, Matsuzawa T (1993) Cryopreservation of asparagus (Asparagus officinalis L.) embryogenic suspension cells and subsequent plant regeneration by vitrification. Plant Sci 91:67–73
Niwata E (1995) Cryopreservation of apical meristems of garlic (Allium sativum L.) and high subsequent plant regeneration. CryoLetters 16:102–107
Ogawa Y, Sakurai N, Oikawa A, Kai K, Morishita Y, Mori K, Moriya K, Fujii F, Aoki K, Suzuki H, Ohta D, Saito K, Shibata D (2012) High-throughput cryopreservation of plant cell cultures for functional genomics. Plant Cell Physiol 53:943–952
Ruzic D, Vujovic T, Cerovic R (2013) Cryopreservation of cherry rootstock Gisela 5 (Prunus cerasus × Prunus canescens) shoot tips by droplet-vitrification technique. J Hortic Res 21:79–85
Sakai A, Engelmann F (2007) Vitrification, encapsulation–vitrification and droplet–vitrification: a review. CryoLetters 28:151–172
Sakai A, Kobayashi S, Oiyama I (1990) Cryopreservation of nuclear cells of navel orange (Citrus sinensis Obs. var. Brasiliensis tanaka) by vitrification. Plant Cell Rep 9:30–33
Sakai A, Matsumoto T, Hirai D, Niino T (2000) Newly developed encapsulation-dehydration protocol for plant cryopreservation. Cryo Lett 21:53–62
Sakai A, Hirai D, Niino T (2008) Development of PVS-based vitrification and encapsulation-vitrification protocols. In: Reed BM (ed) Plant cryopreservation: a practical guide. Springer, Berlin, pp 33–58
Stoffella SL, Posse G, Collantes MB (1998) Variabilidad feno y genotípica de poblaciones de Lotus tenuis que habitan suelos con distintos pH. Ecología Austral 8:57–63
Volk GM, Walters Ch (2006) Plant vitrification solution 2 lowers water content and alters freezing behavior in shoot tips during cryoprotection. Cryobiology 52:48–61
Volk GM, Harris JL, Rotindo KE (2006) Survival of mint shoot tips after exposure to cryoprotectant solution components. Cryobiology 52:305–308
Volk GM, Bonnart R, Shepherd A, Yin Z, Lee R, Polek M, Krueger R (2017) Citrus cryopreservation: viability of diverse taxa and histological observations. Plant Cell Tissue Organ Cult 128:327–334
Watanawikkit P, Tantiwiwat S, Bunn E, Dixon KW, Chayanarit K (2012) Cryopreservation of in vitro-propagated protocorms of Caladenia for terrestrial orchid conservation in Western Australia. Bot J Linn Soc 170:277–282
Withers LA, Engelmann F (1998) In vitro conservation of plant genetic resources. In: Altman A (ed) Biotechnology in agriculture. Marcel Dekker, New York, 57–88
Yamamoto T, Iketani H, Ieki H, Nishizawa Y, Notsuka K, Hibi T, Hayashi T, Matsuta N (2000) Transgenic grapevine plants expressing a rice chitinase with enhance resistance to fungal pathogens. Plant Cell Rep 19:639–646
Acknowledgements
This work was supported by grants from Agencia Nacional de Promoción Científica y Técnica (PICT 2014-3718) and Secretaría General de Ciencia y Técnica-Universidad Nacional del Nordeste (PI A001/14). We extend our sincere appreciation to anonymous reviewers for their critical comments. F. Espasandin, E. Brugnoli, O. Ruiz, and P. Sansberro are members of the Research Council of Argentina (CONICET). G. Ayala and L. Ayala received a scholarship from CONICET.
Author information
Authors and Affiliations
Contributions
FDE, OAR, and PAS conceived and designed the experiments. FDE, EAB, PGA, and LPA performed the research. FDE and PAS wrote the manuscript. All authors read and approved the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Communicated by M. Angeles Revilla.
Rights and permissions
About this article
Cite this article
Espasandin, F.D., Brugnoli, E.A., Ayala, P.G. et al. Long-term preservation of Lotus tenuis adventitious buds. Plant Cell Tiss Organ Cult 136, 373–382 (2019). https://doi.org/10.1007/s11240-018-1522-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11240-018-1522-6