Abstract
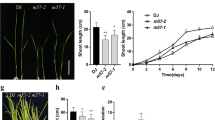
Gibberellic acid (GA) is an important plant hormone mediating plant growth and development throughout the life span. Although many GA biosynthesis genes and signaling components have been revealed, the signal transduction mechanisms from GA perception to physiological actions are still largely unclear. In this study, we investigated the functions of a rice (Oryza sativa) inositol polyphosphate kinase gene (OsIPK2) in rice growth and development, showing that OsIPK2 is a putative new player in GA signaling. OsIPK2 is widely expressed in rice with high accumulation in tender and rapidly dividing tissues. The OsIPK2 protein is mainly localized in the nucleus and plasma membrane. To study the biological roles of OsIPK2 in rice, RNA interference and overexpression transgenic plants were generated. OsIPK2 antisense plants exhibited taller seedling height and lower fertility rate than the wild type, while overexpression lines showed reduced plant height. Microarray and qRT-PCR assays showed that expression levels of several GA-related genes were altered in transgenic plants. Besides, down-regulation of OsIPK2 resulted in hypersensitivity to paclobutrazol (PAC), a GA biosynthesis inhibitor. We also described that the expression of OsIPK2 could be either induced by GA or repressed by PAC. Taken together, these findings suggested that OsIPK2 is likely a negative regulator of GA signaling and involves in modulating shoot elongation and fertility.





Similar content being viewed by others
References
Avila LM, Cerrudo D, Swanton C, Lukens L (2016) Brevis plant1, a putative inositol polyphosphate 5-phosphatase, is required for internode elongation in maize. J Exp Bot 67:1577–1588
Banerjee J, Gantait S, Maiti MK (2017) Physiological role of rice germin-like protein 1 (OsGLP1) at early stages of growth and development in indica rice cultivar under salt stress condition. Plant Cell Tissue Org Cult. doi:10.1007/s11240-017-1270-z**
Bosch D, Saiardi A (2012) Arginine transcriptional response does not require inositol phosphate synthesis. J Biol Chem 287:38347–38355
Cai BD, Yin J, Hao YH, Li YN, Yuan BF, Feng YQ (2015) Profiling of phytohormones in rice under elevated cadmium concentration levels by magnetic solid-phase extraction coupled with liquid chromatography tandem mass spectrometry. J Chromatogr A 1406:78–86
Chen X et al (2015) OsNAC2 encoding a NAC transcription factor that affects plant height through mediating the gibberellic acid pathway in rice. Plant J 82:302–314
Chhun T et al (2007) Gibberellin regulates pollen viability and pollen tube growth in rice. Plant Cell 19:3876–3888
Cho SH, Kang K, Lee SH, Lee IJ, Paek NC (2016) OsWOX3A is involved in negative feedback regulation of the gibberellic acid biosynthetic pathway in rice (Oryza sativa). J Exp Bot 67:1677–1687
Chu CC et al (1975) Establishment of an efficient medium for anther culture of rice through comparative experiments on the nitrogen source. Sci Sin 18:659–668
Cui RF et al (2010) Functional conservation and diversification of class E floral homeotic genes in rice (Oryza sativa). Plant J 61:767–781
Daviere JM, Achard P (2013) Gibberellin signaling in plants. Development 140:1147–1151
Endo-Streeter S, Tsui MK, Odom AR, Block J, York JD (2012) Structural studies and protein engineering of inositol phosphate multikinase. J Biol Chem 287:35360–35369
Fang YJ, Xie KB, Hou X, Hu HH, Xiong LZ (2010) Systematic analysis of GT factor family of rice reveals a novel subfamily involved in stress responses. Mol Genet Genom 283:157–169
Fleet CM, Ercetin ME, Gillaspy GE (2009) Inositol phosphate signaling and gibberellic acid. Plant Signal Behav 4:73–74
Fukao T, Bailey-Serres J (2008) Submergence tolerance conferred by Sub1A is mediated by SLR1 and SLRL1 restriction of gibberellin responses in rice. Proc Nat Acad Sci USA 105:16814–16819
Hedden P, Thomas SG (2012) Gibberellin biosynthesis and its regulation. Biochem J 444:11–25
Hiei Y, Ohta S, Komari T, Kumashiro T (1994) Efficient transformation of rice (Oryza sativa L.) mediated by Agrobacterium and sequence analysis of the boundaries of the T-DNA. Plant J 6:271–282
Ikeda A et al (2001) slender rice, a constitutive gibberellin response mutant, is caused by a null mutation of the SLR1 gene, an ortholog of the height-regulating gene GAI/RGA/RHT/D8. Plant Cell 13:999–1010
Ito H, Fukuda Y, Murata K, Kimura A (1983) Transformation of intact yeast cells treated with alkali cations. J Bacteriol 153:163–168
Itoh H, Ueguchi-Tanaka M, Sato Y, Ashikari M, Matsuoka M (2002) The gibberellin signaling pathway is regulated by the appearance and disappearance of SLENDER RICE1 in nuclei. Plant cell 14:57–70
Itoh H, Shimada A, Ueguchi-Tanaka M, Kamiya N, Hasegawa Y, Ashikari M, Matsuoka M (2005) Overexpression of a GRAS protein lacking the DELLA domain confers altered gibberellin responses in rice. Plant J 44(4):669–679
Kaneko M, Itoh H, Inukai Y, Sakamoto T, Ueguchi-Tanaka M, Ashikari M, Matsuoka M (2003) Where do gibberellin biosynthesis and gibberellin signaling occur in rice plants? Plant J 35:104–115
Kashem MA, Itoh K, Iwabuchi S, Hori H, Mitsui T (2000) Possible involvement of phosphoinositide-Ca2+ signaling in the regulation of alpha-amylase expression and germination of rice seed (Oryza sativa L.). Plant Cell Physiol 41:399–407
Kim E et al (2013) Inositol polyphosphate multikinase is a coactivator for serum response factor-dependent induction of immediate early genes. Proc Nat Acad Sci USA 110:19938–19943
Lee HS, Lee DH, Cho HK, Kim SH, Auh JH, Pai HS (2015) InsP6-sensitive variants of the Gle1 mRNA export factor rescue growth and fertility defects of the ipk1 low-phytic-acid mutation in Arabidopsis. Plant Cell 27:417–431
Liu YG, Ye NH, Liu R, Chen MX, Zhang JH (2010) H2O2 mediates the regulation of ABA catabolism and GA biosynthesis in Arabidopsis seed dormancy and germination. J Exp Bot 61:2979–2990
Ma XD, Ma J, Zhai HH, Xin PY, Chu JF, Qiao YL, Han LZ (2015) CHR729 is a CHD3 protein that controls seedling development in Rice. PLoS ONE 10:e0138934
Qiu DY et al (2007) OsWRKY13 mediates rice disease resistance by regulating defense-related genes in salicylate- and jasmonate-dependent signaling. Mol Plant Microbe Interact 20:492–499
Sasaki A et al (2003) Accumulation of phosphorylated repressor for gibberellin signaling in an F-box mutant. Science 299:1896–1898
Seo M et al (2006) Regulation of hormone metabolism in Arabidopsis seeds: phytochrome regulation of abscisic acid metabolism and abscisic acid regulation of gibberellin metabolism. Plant J 48:354–366
Sheard LB et al (2010) Jasmonate perception by inositol-phosphate-potentiated COI1-JAZ co-receptor. Nature 468:400–405
Shears SB (2004) How versatile are inositol phosphate kinases? Biochem J 377:265–280
Sparvoli F, Cominelli E (2015) Seed biofortification and phytic acid reduction: a conflict of interest for the plant? Plants 4:728–755
Srivastava V, Underwood JL, Zhao S (2017) Dual-targeting by CRISPR/Cas9 for precise excision of transgenes from rice genome. Plant Cell Tiss Organ Cult 129:153–160
Stevenson-Paulik J, Odom AR, York JD (2002) Molecular and biochemical characterization of two plant inositol polyphosphate 6-/3-/5-kinases. J Biol Chem 277:42711–42718
Stevenson-Paulik J, Bastidas RJ, Chiou ST, Frye RA, York JD (2005) Generation of phytate-free seeds in Arabidopsis through disruption of inositol polyphosphate kinases. Proc Nat Acad Sci USA 102:12612–12617
Suzuki M, Tanaka K, Kuwano M, Yoshida KT (2007) Expression pattern of inositol phosphate-related enzymes in rice (Oryza sartiva L.): Implications for the phytic acid biosynthetic pathway. Gene 405:55–64
Swain SM, Reid JB, Kamiya Y (1997) Gibberellins are required for embryo growth and seed development in pea. Plant J 12:1329–1338
Takeuchi K et al (2016) Overexpression of RSOsPR10, a root-specific rice PR10 gene, confers tolerance against drought stress in rice and drought and salt stresses in bentgrass. Plant Cell Tissue Organ Cult 127:35–46
Tan X, Calderon-Villalobos LI, Sharon M, Zheng C, Robinson CV, Estelle M, Zheng N (2007) Mechanism of auxin perception by the TIR1 ubiquitin ligase. Nature 446:640–645
Ueguchi-Tanaka M et al (2005) GIBBERELLIN INSENSITIVE DWARF1 encodes a soluble receptor for gibberellin. Nature 437:693–698
Villasuso AL, Molas ML, Racagni G, Abdala G, Machado-Domenech E (2003) Gibberellin signal in barley aleurone: early activation of PLC by G protein mediates amylase secretion. Plant Growth Regul 41:197
Wang P, Yang QF, Sang SH, Chen Y, Zhong YJ, Wei ZY (2017) Arabidopsis inositol polyphosphate kinase AtIpk2β is phosphorylated by CPK4 and positively modulates ABA signaling. Biochem Biophys Res Commun 490(2):441–446
Xia HJ, Yang G (2005) Inositol 1,4,5-trisphosphate 3-kinases: functions and regulations. Cell Res 15:83–91
Xia HJ, Brearley C, Elge S, Kaplan B, Fromm H, Mueller-Roeber B (2003) Arabidopsis inositol polyphosphate 6-/3-kinase is a nuclear protein that complements a yeast mutant lacking a functional ArgR-Mcm1 transcription complex. Plant Cell 15:449–463
Xu RS, Snyder SH (2013) Gene transcription by p53 requires inositol polyphosphate multikinase as a co-activator. Cell Cycle 12:1819–1820
Xu J et al (2005) A role of Arabidopsis inositol polyphosphate kinase, AtIPK2α, in pollen germination and root growth. Plant Physiol 137:94–103
Xu RS et al (2013) Inositol polyphosphate multikinase is a transcriptional coactivator required for immediate early gene induction. Proc Nat Acad Sci USA 110:16181–16186
Yamaguchi S (2008) Gibberellin metabolism and its regulation. Annu Rev Plant Biol 59:225–251
Yang L, Tang RJ, Zhu JQ, Liu H, Mueller-Roeber B, Xia HJ, Zhang HX (2008) Enhancement of stress tolerance in transgenic tobacco plants constitutively expressing AtIpk2β, an inositol polyphosphate 6-/3-kinase from Arabidopsis thaliana. Plant Mol Biol 66:329–343
Zhan HD, Zhong YJ, Yang ZN, Xia HJ (2015) Enzyme activities of Arabidopsis inositol polyphosphate kinases AtIPK2α and AtIPK2β are involved in pollen development, pollen tube guidance and embryogenesis. Plant J 82:758–771
Zhang ZB, Yang G, Arana F, Chen Z, Li Y, Xia HJ (2007) Arabidopsis inositol polyphosphate 6-/3-kinase (AtIpk2β) is involved in axillary shoot branching via auxin signaling. Plant Physiol 144:942–951
Zhang Y et al (2011) A highly efficient rice green tissue protoplast system for transient gene expression and studying light/chloroplast-related processes. Plant Methods 7(1):30
Acknowledgements
We sincerely thank Prof. Huijun Xia (Wuhan University) for providing helpful advice and experimental conditions; State Key Laboratory of Hybrid Rice for reagents and analytical equipments; Prof. Yongjun Lin (Huazhong Agricultural University) and Yue Li for rice transformation work; Prof. Yuqi Feng (Wuhan University) for quantification of the endogenous ABA content; Prof. Zheng Meng (Institute of Botany, the Chinese Academy of Sciences) for support the pBJWI3 and PU1301 vectors; Prof. Lizhong Xiong (Huazhong Agricultural University) for support the pU1391cGFP vector. We also thank Dr. Xiaolei Fan for northern blot hybridization experiments. This work was supported by the National High Technology Research and Development Program of China (Grant 2006AA10A103).
Author information
Authors and Affiliations
Contributions
YC conceived and designed research. YC, ZW, QY and SS conducted experiments. YC analyzed data and wrote the manuscript.
Corresponding author
Additional information
Communicated by Ming-Tsair Chan.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Chen, Y., Wei, Z., Yang, Q. et al. Rice inositol polyphosphate kinase gene (OsIPK2), a putative new player of gibberellic acid signaling, involves in modulation of shoot elongation and fertility. Plant Cell Tiss Organ Cult 131, 471–482 (2017). https://doi.org/10.1007/s11240-017-1298-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11240-017-1298-0