Abstract
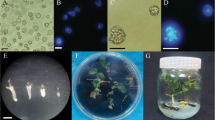
The effects of three periods of exposure (12, 24 and 48 h) to different levels of putrescine (0, 0.2, 0.5, 1.0, 2.0 and 5.0 mg l−1), as well as three incubation periods (24, 48 and 72 h) to different levels of cefotaxime and vancomycin (0, 50, 100, 200 and 500 mg l−1) on microspore embryogenesis of rapeseed cv. ‘Hyola 401’ were assessed. Microspore embryogenesis was enhanced about threefold compared with untreated culture following 48 h treatment with 0.2 mg l−1 putrescine. Putrescine treatment at 0.5 mg l−1 for 48 h effectively induced root formation and increased normal plantlet regeneration by 92 % when microspore-derived embryos (MDEs) were transferred to regeneration medium. The highest embryo yield (184.2 embryos Petri dish−1) was possible when induction medium was supplemented with 50 mg l−1 cefotaxime for 24 h and the highest normal regeneration was observed in cultures exposed to 50 and 100 mg l−1 at all durations tested. More abnormal MDEs (76 and 82 %) were observed when microspores treated with 200 and 500 mg l−1 cefotaxime many of which failed to regenerate normally and resulted in callusing. Vancomycin at 100 mg l−1 during the 48 h exposure increased the number of MDEs (181.6 embryos Petri dish−1) in contrast to untreated cultures (93.6 embryos Petri dish−1) but, normal plantlet regeneration decreased as vancomycin level increased and high callusing (84 and 90 %) was observed with 200 and 500 mg l−1 for 72 h. Microspore embryogenesis and plant regeneration could be improved by putrescine, cefotaxime and vancomycin when appropriate levels and durations of incubation were selected.

Similar content being viewed by others
Abbreviations
- ADC :
-
Arginine decarboxylase
- DH:
-
Doubled haploid
- ELS:
-
Embryo-like structure
- GA3 :
-
Gibberellic acid
- MDE:
-
Microspore-derived embryo
- ODC :
-
Ornithine decarboxylase
References
Ahmadi B, Ghadimzadeh M, Moghaddam AF, Alizadeh K, Teixeira da Silva JA (2012a) Bud length, plating density, and incubation time on microspore embryogenesis in Brassica napus. Int J Veg Sci 18:346–357
Ahmadi B, Alizadeh K, Teixeira da Silva JA (2012b) Enhanced regeneration of haploid plantlets from microspores of Brassica napus L. using bleomycin, PCIB, and phytohormones. Plant Cell Tissue Organ Cult 109:525–533
Ahmadi B, Shariatpanahi ME, Teixeira da Silva JA (2014) Efficient induction of microspore embryogenesis using abscisic acid, jasmonic acid and salicylic acid in Brassica napus L. Plant Cell Tissue Organ Cult 116:343–351
Alcázar R, Altabella T, Marco F, Bortolotti C, Reymond M, Koncz C, Carrasco P, Tiburcio AF (2010) Polyamines: molecules with regulatory functions in plant abiotic stress tolerance. Planta 231:1237–1249
Ardebili SH, Shariatpanahi ME, Amiri R, Emamifar M, Nematzadeh G, Noori SAS, Oroojloo M, Heberle-Bors E (2011) Effect of 2,4-D as a novel inducer of embryogenesis in microspores of Brassica napus L. Czech J Genet Plant Breed 47:114–122
Asif M, Eudes F, Randhawa H, Amundsen E, Yanke J, Spaner D (2013) Cefotaxime prevents microbial contamination and improves microspore embryogenesis in wheat and triticale. Plant Cell Rep 32:1637–1646
Babbar BS, Agarwal PK, Sahay S, Bhojwani SS (2004) Isolated microspore culture of Brassica: an experimental tool for developmental studies and crop improvement. Indian J Biotechnol 3:185–202
Bastola DR, Minocha SC (1995) Increased putrescine biosynthesis through transfer of mouse ornithine decarboxylase cDNA in carrot promotes somatic embryogenesis. Plant Physiol 109:63–71
Bertoldi D, Tassoni A, Martinelli L, Bangi N (2004) Polyamines and somatic embryogenesis in two Vitis vinifera cultivars. Physiol Plant 120:657–666
Bhau BS, Wakhlu AK (2001) Effect of some antibiotics on the in vitro morphogenetic response from callus cultures of Coryphantha elephantidens. Biol Plant 4(1):19–24
Brew-Appiah RAT, Ankrah N, Liu W, Konzak CF, von Wettstein D, Rustgi A (2013) Generation of doubled haploid transgenic wheat lines by microspore transformation. PLoS One. doi:10.1371/journal.pone.0080155
Burgos L, Alburquerque N (2003) Ethylene inhibitors and low kanamycin concentrations improve adventitious regeneration from apricot leaves. Plant Cell Rep 21:1167–1174
Chan SWL (2010) Chromosome engineering: power tools for plant genetics. Trends Biotechnol 28:650–710
Coventry J, Kott LS (1998) Doubled haploid technology for spring and winter Brassica napus, revised ed. OAC Publication, University of Guelph, Ontario, Canada. Technol Bull, p 42
Danilova SA, Dolgikh YI (2004) The stimulatory effect of the antibiotic cefotaxime on plant regeneration in maize tissue culture. Russ J Plant Physiol 51(4):559–562
Dewi IS, Purwoko BS (2008) Role of polyamines in inhibition of ethylene biosynthesis and their effects on rice anther culture development. Indones J Agric Sci 9(2):60–67
Dubas E, Moravčíková J, Libantová J, Matušíková I, Benková E, Żur I, Krzewska M (2014) The influence of heat stress on auxin distribution in transgenic B. napus microspores and microspore-derived embryos. Protoplasma. doi:10.1007/s00709-014-0616-1
Ferrie AMR, Caswell KL (2011) Isolated microspore culture techniques and recent progress for haploid and doubled haploid plant production. Plant Cell Tissue Organ Cult 104:301–309
Ferrie AMR, Keller WA (2007) Optimization of methods for using polyethylene glycol as a non-permeating osmoticum for the induction of microspore embryogenesis in Brassicaceae. In Vitro Cell Dev Biol Plant 43:348–355
Forster BP, Thomas WTB (2005) Doubled haploids in genetics and plant breeding. In: Janis J (ed) Plant Breed Rev. Wiley, New York 25:57–88
Gamborg OL, Miller RA, Ojima L (1968) Nutrient requirements of suspension cultures of soybean root cells. Exp Cell Res 50:151–158
Hammes WP, Neuhaus FC (1974) Mechanism of action of vancomycin—inhibition of peptidoglycan synthesis in Gaffkya homari. Antimicrob Agents Chemother 6:722–728
Holland L, Gemmell JE, Charity JA, Walter C (1997) Foreign gene transfer into Pinus radiate cotyledons by Agrobacterium tomefaciens. N Z J For Sci 27:289–304
Hoseini M, Ghadimzadeh M, Ahmadi B, Teixeira da Silva JA (2014) Effects of ascorbic acid, alpha-tocopherol, and glutathione on microspore embryogenesis in Brassica napus L. In Vitro Cell Dev Biol Plant. doi:10.1007/s11627-013-9579-8
Kackar A, Shekhawat NS (2007) Plant regeneration through embryogenesis and polyamine levels in cultures of grasses of Thar Desert. J Cell Mol Biol 6(2):121–127
Kaur-Sawhney R, Tiburcio AF, Altabella T, Galston AW (2003) Polyamines in plants: an overview. J Cell Mol Biol 2:1–12
Kevers C, Gal NL, Monteiro M, Dommes J, Gaspar T (2000) Somatic embryogenesis for Panax ginseng in liquid cultures: a role for polyamines and their metabolic pathway. Plant Growth Regul 31:209–214
Lichter R (1982) Induction of haploid plants from isolated pollens of Brassica napus. Z Pflanzenphysiol 105:427–434
Liu S, Wang H, Zhang J, Fitt BDL, Xu Z, Evans N, Liu Y, Yang W, Guo X (2005) In vitro mutation and selection of doubled-haploid Brassica napus lines with improved resistance to Sclerotinia sclerotiorum. Plant Cell Rep 24:133–144
Martin-Tanguy J (2001) Metabolism and function of polyamines in plants: recent development (new approaches). Plant Growth Regul 34:135–148
Mittal P, Gosal SS, Senger A, Kumar P (2009) Impact of cefotaxime on somatic embryogenesis and shoot regeneration in sugarcane. Physiol Mol Biol Plants 15(3):257–265
Muñoz-Amatriaín M, Svensson JT, Castillo AM, Close TJ, Vallés MP (2009) Microspore embryogenesis: assignment of genes to embryo formation and green vs. albino plant production. Funct Integr Genomics 9:311–323
Nauerby B, Billing Z, Wyndaele R (1997) Influence of antibiotic timentin on plant regeneration compared to carbenicillin and cefatoxime in concentrations suitable for elimination of Agrobacterium tomefaciens. Plant Sci 123:169–177
Panathula CS, Mahadev MDN, Naidu CV (2014) The stimulatory effect of the antimicrobial agents bavistin, cefotaxime and kanamycin on in vitro plant regeneration of Centella asiatica (L.)—an important antijaundice medicinal plant. Am J Plant Sci 5:279–285
Pius J, George L, Eapen S, Rao PS (1993) Enhanced plant regeneration in pearl millet (Pennisetum americanum) by ethylene inhibitors and cefotaxime. Plant Cell Tissue Organ Cult 32:91–96
Prem D, Gupta K, Agnihotri A (2005) Effect of various exogenous and endogenous factors on microspore embryogenesis in Indian mustard (Brassica juncea L. Czern and Coss). In Vitro Cell Dev Biol Plant 41:266–273
Rakosy-Tican E, Aurori CM, Aurori A (2011) The effects of cefotaxime and silver thiosulphate on in vitro culture of Solanum chacoense. Rom Biotechnol Lett 16(4):6369–6377
Sakhanokho HF, Ozias-Akins P, May OL, Chee PW (2005) Putrescine enhances somatic embryogenesis and plant regeneration in upland cotton. Plant cell Tissue Organ Cult 81:91–95
Sarma KS, Evans NE, Selby C (1995) Effect of carbenicillin and cefotaxime on somatic embryogenesis of Sitka spruce (Picea sitchensis (Bong.) Carr.). J Exp Bot 46:1779–1781
Seguí-Simarro JM, Nuez F (2008) How microspores transform into haploid embryos: changes associated with embryogenesis induction and microspore-derived embryogenesis. Physiol Plant 134:1–12
Shariatpanahi ME, Bal U, Heberle-Bors E, Touraev A (2006) Stresses applied for the re-programming of plant microspores towards in vitro embryogenesis. Physiol Plant 127:519–534
Simmonds JA, Grainger JL (1993) The toxicity of antibiotics to protoplast cultures of Triticum aestivum L. Plant Sci 89:209–214
Swanberg A, Dai W (2008) Plant regeneration of periwinkle (Catharanthus roseus) via organogenesis. Hortic Sci 43(3):832–836
Takasaki T, Hatakeyama K, Ojima K, Watanabe M, Toriyama K, Hinata K (1996) Effects of various factors (hormone combinations, genotypes and antibiotics) on shoot regeneration from cotyledon explants in Brassica rapa L. Plant Tissue Cult Lett 13(2):177–180
Teixeira da Silva JA, Fukai S (2001) The impact of carbencillin, cefotaxime and vancomycin on chrysanthemum and tobacco TCL morphogenesis and Agrobacterium growth. J Appl Hortic 3(1):3–12
Tereso S, Miguel C, Maroco J, Oliveira MM (2006) Susceptibility of embryogenic and organogenic tissues of maritime pine (Pinus pinaster) to antibiotics used in Agrobacterium-mediated genetic transformation. Plant Cell Tissue Organ Cult 87:33–40
Thiruvengadam M, Rekha KT, Jayabalan N, Praveen N, Kim EH, Chung IM (2013) Effect of exogenous polyamines enhances somatic embryogenesis via suspension cultures of spine guard (Momordica dioidca Roxb. ex. Wild). Australian. J Crop Sci 7(3):446–453
Touraev A, Vicente O, Heberle-Bors E (1997) Initiation of microspore embryogenesis by stress. Trends Plant Sci 2:297–302
Urano K, Hobo T, Shinozaki K (2005) Arabidopsis ADC genes involved in polyamine biosynthesis are essential for seed development. FEBS Lett 579:1557–1564
Wu X-B, Wang J, Liu J-H, Deng X-X (2009) Involvement of polyamine biosynthesis in somatic embryogenesis of Valencia sweet orange (Citrus sinensis) induced by glycerol. J Plant Physiol 166:52–62
Yu Y, Wei ZM (2007) Influences of cefotaxime and carbenicillin on plant regeneration from wheat mature embryos. Biol Plant 52(3):553–556
Zhao J, Sun MX (2005) Influence of exogenous auxin on microspore embryogenesis in tobacco. Wuhan Bot Res 23(6):519–523
Żur I, Dubas E, Krzewska M, Sanchez-Díaz RA, Castillo AM, Vallés MP (2014) Changes in gene expression patterns associated with microspore embryogenesis in hexaploid triticale (× Triticosecale Wittm.). Plant Cell Tissue Organ Cult 116:261–267
Acknowledgments
This research was supported by grants from Agricultural Biotechnology Research Institute of Iran (ABRII) Project No. 1-05-05-8601.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ahmadi, B., Shariatpanahi, M.E., Ojaghkandi, M.A. et al. Improved microspore embryogenesis induction and plantlet regeneration using putrescine, cefotaxime and vancomycin in Brassica napus L.. Plant Cell Tiss Organ Cult 118, 497–505 (2014). https://doi.org/10.1007/s11240-014-0501-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11240-014-0501-9