Abstract
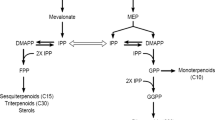
In Catharanthus roseus cell cultures, the monoterpenoid pathway has been shown to be a limiting factor in terpenoid indole alkaloid (TIA) production. This could be due to competition at the level of isopentenyl diphosphate::dimethylallyl diphosphate (C5) which leads to the biosynthesis of different terpenoid groups. For future engineering of the terpenoid pathway, chemical characterization of C. roseus cell cultures is a necessity. Therefore, in this study nine C. roseus cell suspension lines were characterized by analyzing the levels of the major terpenoids derived from different biosynthetic pathways which may compete for the same precursors; TIA (monoterpenoid, C10), carotenoids (tetraterpenoid, C40), and sterols (triterpenoid, C30). Among the cell lines, CRPP (S) was the most promising TIA-producing cell line which provided more TIA [24 μmol g−1 dry weight (DW)] than carotenoids (15 μmol g−1 DW) and sterols (2 μmol g−1 DW). However, when considering the distribution of the isopentenyl-precursor (C5), the carotenoids which assemble from 8× C5 represent twofold more C5-units (122 μmol g−1 DW) than the TIA in this cell line. In the CRPP (G), A12A2 (G), and A12A2 (S) cell lines, the C5 distribution was predominant toward carotenoid biosynthesis as well, resulting in a relatively high accumulation of carotenoids. The geranylgeranyl diphosphate (C20) pathway toward carotenoid production is therefore considered competitive toward TIA biosynthesis. For channeling more precursors to the TIA, the branch point for C10 and C20 seems an interesting target for metabolic engineering. Using principal component analysis of the chromatographic data, we characterized the cell lines chemically based on their metabolite levels. The information on the metabolic composition of C. roseus cell cultures is useful for developing strategies to engineer the metabolic pathways and for selection of cell lines for future studies.







Similar content being viewed by others
References
Amini A, Glévarec G, Andreu F, Rideau M, Crèche J (2009) Low levels of gibberellic acid control the biosynthesis of ajmalicine in Catharanthus roseus cell suspension cultures. Planta Med 75:187–191
Ben-Amotz A, Lers A, Avron M (1988) Stereoisomers of β-carotene and phytoene in the alga Dunaliella bardawil. Plant Physiol 86:1286–1291
Bino RJ, de Vos RCH, Lieberman M, Hall RD, Bovy A, Jonker HH, Tikunov Y, Lommen A, Moco S, Levin I (2005) The light-hyperresponsive high pigment-2 dg mutation of tomato: alterations in the fruit metabolome. New Phytol 166:427–438
Blom TJM, Sierra M, van Vliet TB, Franke-van Dijk MEI, de Koning P, van Iren F, Verpoorte R, Libbenga KR (1991) Uptake and accumulation of ajmalicine into isolated vacuoles of cultured cells of Catharanthus roseus (L.) G. Don. and its conversion into serpentine. Planta 183:170–177
Chung IM, Kim EH, Li M, Peebles CAM, Jung WS, Song HK, Ahn JK, San KY (2011) Screening 64 cultivars Catharanthus roseus for the production of vindoline, catharanthine, and serpentine. Biotechnol Prog 27:937–943
Collu G, Garcia AA, van der Heijden R, Verpoorte R (2002) Activity of the cytochrome P450 enzyme geraniol 10-hydroxylase and alkaloid production in plant cell cultures. Plant Sci 162:165–172
Contin A, Collu G, van der Heijden R, Verpoorte R (1999) The effects of phenobarbital and ketoconazole on the alkaloid biosynthesis in Catharanthus roseus cell suspension cultures. Plant Physiol Biochem 37:139–144
Courdavault V, Burlat V, St-Pierre B, Giglioli-Guivarc’h N (2005) Characterisation of CaaX-prenyltransferases in Catharanthus roseus: relationships with the expression of genes involved in the early stages of monoterpenoid biosynthetic pathway. Plant Sci 168:1097–1107
Dutta A, Batra J, Pandey-Rai S, Singh D, Kuar S, Sen J (2005) Expression of terpenoid indole alkaloid biosynthethic pathway genes corresponds to accumulation of related alkaloids in Catharanthus roseus (L.) G. Don. Planta 220:376–383
El-Sayed M, Verpoorte R (2002) Effect of phytohormones on growth and alkaloid accumulation by a Catharanthus roseus cell suspension cultures fed with alkaloid precursors tryptamine and loganin. Plant Cell Tissue Organ Cult 68:265–270
El-Sayed M, Verpoorte R (2007) Catharanthus terpenoid indole alkaloids: biosynthesis and regulation. Phytochem Rev 6:277–305
Gamborg OL, Miller RA, Ojima K (1968) Nutrient requirements of suspension cultures of soybean root cells. Exp Cell Res 50:155–158
Glenn WS, Runguphan W, O’Connor SE (2013) Recent progress in the metabolic engineering of alkaloids in plant systems. Curr Opin Biotechnol 24:354–365
Hallard DAC (2000) Transgenic plant cells for the production of indole alkaloids. PhD Thesis, Leiden University, The Netherlands. Chapter 7, Extraction and liquid chromatography photodiode array detection tandem mass spectrometry analysis of Catharanthus alkaloids, pp 88–109. ISBN 9074538495
Hemmerlin A, Hoeffler JF, Meyer O, Tritsch D, Kagan IA, Grosdemange-Billiard C, Rohmer M, Bach TJ (2003) Cross-talk between the cytosolic mevalonate and the plastidial methylerythritol phosphate pathways in tobacco bright yellow-2 cells. J Biol Chem 278:26666–26676
Laule O, Fürholz A, Chang H-S, Zhu T, Wang X, Heifetz PB, Gruissem W, Lange BM (2003) Crosstalk between cytosolic and plastidial pathways of isoprenoid biosynthesis in Arabidopsis thaliana. Proc Natl Acad Sci USA 100:6866–6871
Moreno PRH, van der Heijden R, Verpoorte R (1993) Effect of terpenoid precursor feeding and elicitation on formation of indole alkaloids in cell suspension cultures of Catharanthus roseus. Plant Cell Rep 12:702–705
Moreno PRH, van der Heijden R, Verpoorte R (1995) Cell and tissue cultures of Catharanthus roseus: a literature survey II. Updating from 1988 to 1993. Plant Cell Tissue Organ Cult 42:1–25
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15:473–497
Murata J, De Luca V (2005) Localization of tabersonine 16-hydroxylase and 16-OH tabersonine-16-O-methyltransferase to leaf epidermal cells defines them as a major site of precursor biosynthesis in the vindoline pathway in Catharanthus roseus. Plant J 44:581–594
Mustafa NR, Verpoorte R (2007) Phenolic compounds in Catharanthus roseus. Phytochem Rev 6:243–258
Mustafa NR, Kyong Kim Hye, Choi YH, Verpoorte R (2009) Metabolic changes of salicylic acid-elicited Catharanthus roseus cell suspension cultures monitored by NMR-based metabolomics. Biotechnol Lett 31:1967–1974
Mustafa NR, de Winter W, van Iren F, Verpoorte R (2011) Initiation, growth and cryopreservation of plant cell suspension cultures. Nat Protoc 6:715–742
Namitha KK, Negi PS (2010) Chemistry and biotechnology of carotenoids. Crit Rev Food Sci Nutr 50:728–760
O’Connor SE (2012) Strategies for engineering plant natural products: the iridoid-derived monoterpene indole alkaloids of Catharanthus roseus. Methods Enzymol 515:189–206
Pati PK, Kaur J, Singh P (2011) A liquid culture system for shoot proliferation and analysis of pharmaceutically active constituents of Catharanthus roseus (L.) G. Don. Plant Cell Tissue Organ Cult 105:299–307
Pietrosiuk A, Furmanowa M, Łata B (2007) Catharanthus roseus: micropropagation and in vitro techniques. Phytochem Rev 6:459–473
Rohmer M (1999) The discovery of a mevalonate-independent pathway for isoprenoid biosynthesis in bacteria, algae and higher plants. Nat Prod Rep 16:565–574
Saimoto H, Nakagawa S, Kobayashi M, Fujioka S, Barreto MCC, Sakurai A, Syono K (1990) Endogenous levels of gibberellins, IAA and cytokinins in Catharanthus crown gall tissues of different tumor types. Plant Cell Physiol 31:365–370
Salim V, De Luca V (2013) Towards complete elucidation of monoterpene indole alkaloid biosynthesis pathway: Catharanthus roseus as a pioneer system. In: Giglioli-Guivarc’h N (ed) Advances of botanical research—new light on alkaloid biosynthesis and future prospects. Academic Press, London, pp 1–37
Schuhr CA, Radykewicz T, Sagner S, Latzel C, Zenk MH, Arigoni D, Bacher A, Rohdich F, Eisenreich W (2003) Quantitative assessment of crosstalk between the two isoprenoid biosynthesis pathways in plants by NMR spectroscopy. Phytochem Rev 2:3–16
St-Pierre B, Vazquez-Flota F, De Luca V (1999) Multicellular compartmentation of Catharanthus roseus alkaloid biosynthesis predicts intercellular translocation of pathway intermediate. Plant Cell 11:887–900
Suzuki H, Inoue T, Fujioka S, Saito T, Takatsuto S, Yokota T, Murofushi N, Yanagisawa T, Sakurai A (1995) Conversion of 24-methylcholesterol to 6-oxo-24-methylcholestanol, a putative intermediate of the biosynthesis of brassinosteroids, in cultured cells of Cathanranthus roseus. Phytochemistry 40:1391–1397
Taylor RF, Farrow PE, Yelle LM, Harris JC, Marenchic IG (1990) Advances in HPLC and HPLC-MS of carotenoids and retinoids. In: Krinsky NI, Mathews-Roth MM, Taylor RF (eds) Carotenoids: chemistry and biology. Plenum Press, New York, pp 105–123
Tikhomiroff C, Jolicoeur M (2002) Screening of Catharanthus roseus secondary metabolites by high-performance liquid chromatography. J Chromatogr A 955:87–93
van der Heijden R, Jacobs DI, Snoeijer W, Hallard D, Verpoorte R (2004) The Catharanthus alkaloids: pharmacognosy and biotechnology. Curr Med Chem 11:607–628
Verpoorte R, Contin A, Memelink J (2002) Biotechnology for the production of plant secondary metabolites. Phytochem Rev 1:13–25
Whitmer S, Canel C, Hallard D, Goncalves C, Verpoorte R (1998) Influence of precursor availability on alkaloid accumulation by transgenic cell line of Catharanthus roseus. Plant Physiol 116:853–857
Whitmer S, van der Heijden R, Verpoorte R (2002) Effect of precursor feeding on alkaloid accumulation by a strictosidine synthase over-expressing transgenic cell line S1 of Catharanthus roseus. Plant Cell Tissue Organ Cult 69:85–93
Zhao J, Verpoorte R (2007) Manipulating indole alkaloid production by Catharanthus roseus cell cultures in bioreactors: from biochemical processing to metabolic engineering. Phytochem Rev 6:435–457
Zhou ML, Shao JR, Tang YX (2009) Production and metabolic engineering of terpenoid indole alkaloids in cell cultures of the medicinal plant Catharanthus roseus (L.) G. Don (Madagascar periwinkle). Biotechnol Appl Biochem 52:313–323
Acknowledgments
The authors thank Mr. L. Verhagen (Prisna B.V., Leiden, The Netherlands) for assistance with sterol analysis. The authors also thank the Ministry of Education, Malaysia and University of Malaya, Kuala Lumpur, Malaysia for financial support of Mohd Zuwairi Saiman. This research was funded by the IBOS-ACTS program as coordinated by NWO.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Saiman, M.Z., Mustafa, N.R., Pomahočová, B. et al. Analysis of metabolites in the terpenoid pathway of Catharanthus roseus cell suspensions. Plant Cell Tiss Organ Cult 117, 225–239 (2014). https://doi.org/10.1007/s11240-014-0435-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11240-014-0435-2