Abstract
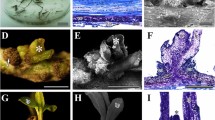
The capacity of regeneration of adventitious shoots from leaf explants was studied in sour cherry ‘Čačanski Rubin’ (Prunus cerasus L.) and cherry rootstock Gisela 5 (P. cerasus × P. canescens). Regeneration assay included thirty different combinations of plant growth regulators. 6-benzyladenine (BA) and thidiazuron (TDZ) were applied either individually or each combined with different concentrations of indole-3-butyric acid, α-naphthaleneacetic acid (NAA) and 2,4-dichlorophenoxyacetic acid (2,4-D). ‘Čačanski Rubin’ showed higher regeneration capacity in comparison with Gisela 5 regarding the total number of treatments inducing regeneration as well as the highest frequency of regeneration achieved. In both genotypes, 8.9 μM BA was more effective than both 4.5 and 9.0 μM TDZ in inducing adventitious regeneration, but only when combined with auxins. The highest frequency of regeneration (20.8 %) in ‘Čačanski Rubin’ was achieved on medium supplemented with 8.9 μM BA combined with 5.4 μM NAA, while in Gisela 5 the highest value (8.3 %) was obtained when BA was combined with 4.5 μM 2,4-D. Flow cytometry combined with 4′-6-diamidino-2-phenylindole staining was employed to estimate DNA ploidy levels and relative nuclear DNA content in adventitious regeneration-derived shoots, in vitro shoots of axillary origin and in vivo control plants from open field. No significant differences in nuclear DNA content were detected among plants of different origin. Chromosome counting in root tip meristems also showed normal tetraploid chromosome number (2n = 4x = 32) in ‘Čačanski Rubin’ shoots and normal triploid chromosome number (2n = 3x = 24) in Gisela 5 shoots regenerated in vitro. The results obtained suggest that no major genetic instability occurred during adventitious regeneration under the described experimental conditions.




Similar content being viewed by others
Abbreviations
- BA:
-
6-benzyladenine
- CV:
-
Coefficient of variation
- 2,4-D:
-
2,4-dichlorophenoxyacetic acid
- DAPI:
-
4′-6-diamidino-2-phenylindole
- G0/G1 :
-
Resting phase of normal cell cycle
- GA3 :
-
Gibberellic acid
- IBA:
-
Indole-3-butyric acid
- MS:
-
Murashige and Skoog (1962)
- NAA:
-
α-naphthaleneacetic acid
- PGRs:
-
Plant growth regulators
- TDZ:
-
Thidiazuron (N-pheny-N′-1,2,3-thidiazol-5-ylurea)
References
Al-Zahim MA, Ford-Lloyd BV, Newbury HJ (1999) Detection of somaclonal variation in garlic (Allium sativum L.) using RAPD and cytological analysis. Plant Cell Rep 18:473–477
Arumuganathan K, Earle ED (1991) Estimation of nuclear DNA content of plants by flow cytometry. Plant Mol Biol Rep 9:229–233
Bassi G, Cassio F (1994) Simplified protocol for in vitro shoot regeneration from leaves of Prunus domestica L. (cv Susina di Dro). In: Schmidt H, Kellerhals M (eds) Progress in temperate fruit breeding. Kluwer, The Netherlands, pp 361–363
Bhagwat B, Lane DW (2004) In vitro shoot regeneration from leaves of sweet cherry (Prunus avium) Lapins and Sweetheart. Plant Cell Tissue Organ Cult 78:173–181
Bortiri E, Oh SH, Jiang J, Baggett S, Granger A, Weeks C, Buckingham M, Potter D, Parfitt DE (2001) Phylogeny and systematics of Prunus (Rosaceae) as determined by sequence analysis of ITS and the chloroplast trnL-trnF spacer DNA. Syst Bot 26:797–807
Canli FA, Tian L (2008) In vitro regeneration from stored mature cotyledons of sweet cherry (Prunus avium L.) cultivars. Sci Hortic-Amsterdam 116:34–40
Doležel J (1997) Application of flow cytometry for the study of plant genomes. J Appl Genet 38:285–302
Doležel J, Bartoš J (2004) Plant DNA flow citometry and estimation of nuclear genome size. Ann Bot-London 95:99–110
Elmaghrabi A, Ochatt S (2006) Isoenzymes and flow cytometry for the assessment of true-to-typeness of calluses and cell suspensions of barrel medic prior to regeneration. Plant Cell Tissue Organ Cult 85:31–43
Emshwiller E (2002) Ploidy levels among species in the ‘Oxalis tuberosa Alliance’ as inferred by flow cytometry. Ann Bot-London 89:741–753
Escalettes V, Dosba F (1993) In vitro adventitious shoot regeneration from leaves of Prunus spp. Plant Sci 90:201–209
Espinosa AC, Pijut PM, Michler CH (2006) Adventitious shoot regeneration and rooting of Prunus serotina in vitro culture. HortScience 41:193–201
Fiuk A, Bednarek PT, Rybczyński JJ (2010) Flow cytometry, HPLC-RP, and metAFLP analyses to assess genetic variability in somatic embryo-derived plantlets of Gentiana pannonica Scop. Plant Mol Biol Rep 28:413–420
Gajdošova A, Ostrolucka MG, Libiakova G, Ondruškova E (2007) Protocol for micropropagation of Vaccinium vitis-idea L. In: Jain SM, Haggman H (eds) Protocols for micropropagation of woody trees and fruits. Springer, Berlin, pp 457–464
Gentile A, Monticelli S, Damiano C (2002) Adventitious shoot regeneration in peach (Prunus persica L. Batsch). Plant Cell Rep 20:1011–1016
Ghimire BK, Yu CY, Chung I-M (2012) Direct shoot organogenesis and assessment of genetic stability in regenerants of Solanum aculeatissimum Jacq. Plant Cell Tissue Organ Cult 108:455–464
Grant NJ, Hammat N (2000) Adventitious shoot development from wild cherry (Prunus avium L.) leaves. New For 20:287–295
Grattapaglia D, Bradshaw HD (1994) Nuclear DNA content of commercially important Eucalyptus species and hybrids. Can J For Res 24:1074–1078
Hammerschlag FA, Bauchan G, Scorza R (1985) Regeneration of peach plants from callus derived from immature embryos. Theor Appl Genet 70:248–251
Iantcheva A, Vlahova M, Trinh TH, Brown SC, Slater A, Elliott MC, Atanassov A (2001) Assessment of polysomaty, embrio formation and regeneration in liquid media for various species of diploid Medicago. Plant Sci 160:621–627
Jin S, Mushke R, Zhu H, Tu L, Lin Z, Zhang Y, Zhang X (2008) Detection of somaclonal variation of cotton (Gossypium hirsutum) using cytogenetics, flow cytometry and molecular markers. Plant Cell Rep 27:1303–1316
Kuksova VB, Piven NM, Gleba YY (1997) Somaclonal variation and in vitro induced mutagenesis in grapevine. Plant Cell Tissue Organ Cult 49:17–27
Landi L, Mezzetti B (2006) TDZ, auxin and genotype effects on leaf organogenesis in Fragaria. Plant Cell Rep 25:281–288
Larkin PJ, Scowcroft WR (1981) Somaclonal variation: a novel source of variability from cell cultures for plant improvement. Theor Appl Genet 60:197–214
Lema-Rumińska J (2011) Flow cytometric analysis of somatic embryos, shoots, and calli of the cactus Copiapoa tenuissima Ritt. forma monstruosa. Plant Cell Tissue Organ Cult 106:531–535
Loureiro JCM (2007) Flow cytometric approaches to study plant genomes. PhD Dissertation, Department of Biology, University of Aveiro, p 268
Loureiro J, Pinto G, Lopes T, Doležel J, Santos C (2005) Assesment of ploidy stability in the somatic embryogenesis process in Quercus suber L. using flow cytometry. Planta 221:815–822
Mallón R, Rodríguez-Oubiña J, González ML (2010) In vitro propagation of the endangered plant Centaurea ultreiae: assessment of genetic stability by cytological studies, flowcytometry and RAPD analysis. Plant Cell Tissue Organ Cult 101:31–39
Mante S, Scorza R, Cordts JM (1989) Plant regeneration from cotyledons of Prunus persica, Prunus domestica and Prunus cerasus. Plant Cell Tissue Organ Cult 19:1–11
Matt A, Jehle AJ (2005) In vitro plant regeneration from leaves and internode sections of sweet cherry cultivars (Prunus avium L.). Plant Cell Rep 24:468–476
Murashige T, Skoog F (1962) A resived medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plantarum 15:473–497
Nehra NS, Kartha KK, Stushnoff C, Giles KL (1992) The influence of plant growth regulator concentrations and callus age on somaclonal variation in callus culture regenerants of strawberry. Plant Cell Tissue Organ Cult 29:257–268
Nowak B, Miczynski K, Hudy L (2004) Sugar uptake and utilisation during adventitious bud differentiation on in vitro leaf explants of Wegierka Zwykla plum (Prunus domestica). Plant Cell Tissue Organ Cult 76:255–260
Pascual L, Marin JA (2005) A liquid 2,4-D pulse increased shoot and root regeneration from leaf explants of adult Prunus rootstocks. Sci Hortic-Amsterdam 106:582–592
Pinto G, Loureiro J, Lopes T, Santos C (2004) Analysis of the genetic stability of Eucalyptus globulus Labill. somatic embryos by flow cytometry. Theor Appl Genet 109:580–587
Ružić Đ, Cerović R, Bošković R (1991) The assessment of somaclonal variation in sour cherry cv Šumadinka regenerated from leaf explants. Fruit Sci Rep 18:155–162
Salesses G, Bonherta A (1993) Meiotic behaviour of hibrids between Prunus cerasifera, P. spinosa and P. persica: an approach to the peach-plum genome relationship. Cytologia 58:257–262
Tang H, Ren Z, Reustle G, Krczal G (2002) Plant regeneration from leaves of sweet and sour cherry cultivars. Sci Hortic-Amsterdam 93:235–244
Tang Z-Q, Chen D-L, Song Z-J, He Y-C, Cai D-T (2010) In vitro induction and identification of tetraploid plants of Paulownia tomentosa. Plant Cell Tissue Organ Cult 102:213–220
Tremblay L, Levasseur C, Tremblay FM (1999) Frequency of somaclonal variation in plants of black spruce (Picea mariana, Pinaceae) and white spruce (P. glauca, Pinaceae) derived from somatic embryogenesis and identification of some factors involved in genetic instability. Am J Bot 86:1373–1381
Vargas TE, Xena N, Vidal MC, Oropeza M, Garcia E (2008) Genetic stability of Solanum tuberosum L. cv. Desiree plantlets obtained from embryogenic cell suspension cultures. Interciencia 33:213–218
Vujović T, Ružić Đ, Cerović R, Šurlan-Momirović G (2010) Adventitious regenerationin blackberry (Rubus fruticosus L.) and assessment of genetic stability in regenerants. Plant Growth Regul 61:265–275
Acknowledgments
This work has been subsidized by Ministry of Education and Science of the Republic of Serbia (projects no. TR-20013A and TR-31064).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Vujović, T., Cerović, R. & Ružić, D. Ploidy level stability of adventitious shoots of sour cherry ‘Čačanski Rubin’ and Gisela 5 cherry rootstock. Plant Cell Tiss Organ Cult 111, 323–333 (2012). https://doi.org/10.1007/s11240-012-0197-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11240-012-0197-7