Abstract
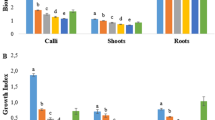
Patchouli is an aromatic shrub of commercial interest because its essential oil is rich in patchoulol. This study aimed to evaluate the effect of growth regulators on callus production, analyze the essential oil production in calli and evaluate metabolic differences between callus, in vitro grown-plantlets and greenhouse-grown plants in three different accessions of patchouli. Calli were induced from leaf explants on media supplemented with 2,4-dichlorophenoxyacetic acid (2,4-D) in combination with 6-benzyladenine (BA). The largest size calli from different accessions were obtained in the presence of the two plant growth regulators (PGRs). For accession POG014, presence of 0.022 mg l−1 2,4-D plus 0.022 mg l−1 BA were optimum. For accession POG021, presence of 0.110 mg l−1 2,4-D plus 0.022 mg l−1 of BA induced the largest callus, whereas for accession POG002, 0.022 mg l−1 2,4-D and 0.225 mg l−1 BA, as well as 0.11 mg l−1 2,4-D and 0.022 mg l−1 BA promoted the development of largest callus. Among all accessions, peroxidase activity was highest in organogenic calli of accession POG014, whereas, polyphenol oxidase activity was highest in in vitro-grown plantlets of accession POG021. Biochemical variables differed significantly among the treatments, with the exception of total sugar levels. The highest concentrations of total sugars were observed in the calli and in vitro-grown plantlets of POG014 and POG021. Essential oils were not detected in callus tissues.



Similar content being viewed by others
Abbreviations
- 2,4-D:
-
2,4-dichlorophenoxyacetic acid
- BA:
-
6-benzyladenine
- AGB:
-
Active germoplasm bank
- GC–MS:
-
Gas chromatograph–mass spectrometer
- MS:
-
Murashige and Skoog culture medium
- POX:
-
Peroxidase
- PPO:
-
Polyphenol oxidase
- PGRs:
-
Plant growth regulators
References
Abbasi BH, Khan M, Guo B, Bokhari SA, Khan MA (2010) Efficient regeneration and antioxidative enzyme activities in Brassica rapa var. turnip. Plant Cell Tiss Organ Cult. doi:10.1007/s11240-010-9872-8
Adams RP (2007) Identification of essential oil components by gas chromatograph/mass spectroscopy, 4th edn. Allured Publishing Corporation, Illinois
Bates LS, Waldern RP, Teare ID (1973) Rapid determination of free proline for water stress studies. Plant Soil 39:205–207. doi:10.1007/BF00018060
Bizzo HR (2009) Essential oils in Brazil: general aspects, development and prospects. Quím Nova 32(3):588–594
Blanc G, Lardet L, Martin A, Jacob JL, Jacob JL, Carron MP (2002) Differential carbohydrate metabolism conducts morphogenesis in embryogenic callus of Hevea brasiliensis (Müll. Arg.). J Exp Bot 53(373):1453–1462. doi:10.1093/jexbot/53.373.1453
Blazquez S, Olmos E, Hernández JA, Fernández-García N, Piqueras A (2009) Somatic embryogenesis in saffron (Crocus sativus L.). Histological differentiation and implication of some components of the antioxidant enzymatic system. Plant Cell Tiss Organ Cult 97:49–57. doi:10.1007/s11240-009-9497-y
Bonfill M, Cusidó RM, Palazón J, Canut E, Piñol MT, Morales C (2003) Relationship between peroxidase activity and organogenesis in Panax ginseng calluses. Plant Cell Tissue Organ Cult 73:37–41. doi:10.1023/A:1022615112745
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248
Bunrathep S, Lockwood GB, Songsak T, Ruangrungsi N (2006) Chemical constituents from leaves and cell cultures of Pogostemon cablin and use of precursor feeding to improve patchouli alcohol level. Sci Asia 32:293–296. doi:10.2306/scienceasia1513-1874.2006.32.293
Costa AG (2008) Desenvolvimento vegetativo, rendimento e composição do óleo essencial de patchouli após adubação nitrogenada. Dissertation, Universidade Federal do Paraná
Dimassi-Theriou K, Bosabalidis AM (1997) Effects of light, magnesium and sucrose on leaf anatomy, photosynthesis, starch and total sugar accumulation, in kiwifruit cultured in vitro. Plant Cell, Tissue Organ Cult 47:127–134. doi:10.1007/BF02318948
Dubois M, Gilles KA, Hamilton JK, Rebers PA, Smith F (1956) Colorimetric method for determination of sugars and related substances. Anal Chem 28(3):350–356
Endress V, Barriuso J, Ruperez P, Martin JP, Blazquez A, Villalobos N, Guerra H, Martin L (2009) Differences in cell wall polysaccharide composition between embryogenic and non-embryogenic calli of Medicago arborea L. Plant Cell Tiss Organ Cult 97:323–329. doi:10.1007/s11240-009-9531-0
Gaspar T, Franck T, Bisbis B, Kevers C, Jouve L, Hausman JF, Dommes J (2002) Concepts in plant stress physiology: application to plant tissue cultures. Plant Growth Regul 37:263–285. doi:10.1023/A:1020835304842
Głowacka K, Jezowski S, Kaczmarek Z (2010) The effects of genotype, inflorescence developmental stage and induction medium on callus induction and plant regeneration in two Miscanthus species. Plant Cell Tiss Organ Cult 102:79–86. doi:10.1007/s11240-010-9708-6
Guenther E (1972) The essential oils: volume three–individual essential oils of the plant families Rutaceae and Labiatae. Krieger Publishing Company, Malabar
He Y, Guo X, Lu R, Niu B, Pasapula V, Hou P, Cai F, Xu Y, Chen F (2009) Changes in morphology and biochemical indices in browning callus derived from Jatropha curcas hypocotyls. Plant Cell Tiss Organ Cult 98:11–17. doi:10.1007/s11240-009-9533-y
Hsu HC, Yang WC, Tsai WJ, Chen CC, Huang HY, Tsai YC (2006) α-Bulnesene, a novel PAF receptor antagonist isolated from Pogostemon cablin. Biochem Biophys Res Commun 345(3):1033–1038. doi:10.1016/j.bbrc.2006.05.006
Jeyaseelan M, Rao MV (2005) Biochemical studies of embryogenic and non-embryogenic callus of Cardiospermum halicacabum L. Indian J Exp Biol 43(6):555–560
Kumar V, Chawla HS (2007) Organogenic regeneration from different explants of patchouli (Pogostemon cablin). Indian J Genet Plant Breed 67(2):187–189
Li Z, Mize K, Campbell F (2010) Regeneration of daylily (Hemerocallis) from young leaf segments. Plant Cell Tiss Organ Cult 102:199–204. doi:10.1007/s11240-010-9722-8
Lima EC, Paiva R, Nogueira RC, Soares FP, Emrich EB, Silva AAN (2008) Callus induction in leaf segments of Croton urucurana Baill. Ciênc Agrotec 32(1):17–22. doi:10.1590/S1413-70542008000100002
Marimuthu S, Kumar RR (2001) Physiological and biochemical responses of micropropagated tea plants. In Vitro Cell Dev Biol Plant 37:618–621. doi:10.1007/s11627-001-0108-9
Martin KP (2004) Plant regeneration protocol of medicinally important Andrographis paniculata (Burm. F.) Wallich ex nees via somatic embryogenesis. In Vitro Cell Dev Biol Plant 40:204–209. doi:10.1079/IVP200352
Mayer AM (2006) Polyphenol oxidases in plants and fungi: Going places? A review. Phytochem 67(2):2318–2331. doi:10.1016/j.phytochem.2006.08.006
Miller GL (1959) Use of dinitosalicylic acid reagent for the determination of reducing sugar. Anal Chem 31:426–428
Mukherjee A, Debata BK, Mukherjee PS, Malik SK (2001) Morphohistobiochemical characteristics of embryogenic and nonembryogenic callus cultures of sweet potato (Ipomoea batatas L.). Cytobios 106(412):113–124
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plantarum 15:473–479. doi:10.1111/j.1399-3054.1962.tb08052.x
Niemenak N, Katja Saare-Surminski KS, Rohsius C, Ndoumou DO, Lieberei R (2008) Regeneration of somatic embryos in Theobroma cacao L. in temporary immersion bioreactor and analyses of free amino acids in different tissues. Plant Cell Rep 27:667–676. doi:10.1007/s00299-007-0497-2
Nogueira RC, Paiva R, Oliveira LM, Soares GA, Soares FP, Castro AHF, Paiva PDO (2007) Callus induction in leaf explants of murici-pequeno (Byrsonima intermedia A. Juss). Ciênc Agrotec 31(2):366–370. doi:10.1590/S1413-70542007000200015
Oropeza M, Marcano AK, De García E (2001) Proteins related with embryogenic potential in callus and cell suspensions of sugarcane (Saccharum sp.). In Vitro Cell Dev Biol Plant 37:211–216. doi:10.1079/IVP2000123
Park S-Y, Cho, H-M, Moon, H-K, Kim, Y-W, Paek K-Y (2010) Genotypic variation and aging effects on the embryogenic capability of Kalopanax septemlobus. Plant Cell Tiss Organ Cult. doi:10.1007/s11240-010-9862-x
Piza IMT, Lima GPP, Brasil OG (2003) Peroxidase activity and protein levels in micropropagated pineapple plants in saline. Rev Bras Agrociênc 9(4):361–366
Pretto FR, Santarém ER (2000) Callus formation and plant regeneration from Hypericum perforatum leaves. Plant Cell Tissue Organ Cult 62:107–113. doi:10.1023/A:1026534818574
Rathod Z, Saxena OP (2007) Biochemical profile of in vivo and in vitro produced Bougainvillea spectabilis L. Indian J Plant Physiol 12(3):234–238
Reuveni R (1995) Biochemical markers for disease resistance. In: Sing RP, Sing US (eds) Molecular methods in plant pathology. CRC Press, New York, pp 99–114
Rodríguez A, Cervera M, Peris JE, Peña L (2008) The same treatment for transgenic shoot regeneration elicits the opposite effect in mature explants from two closely related sweet orange (Citrus sinensis (L.) Osb.) genotypes. Plant Cell Tiss Organ Cult 93:97–106. doi:10.1007/s11240-008-9347-3
Rogalski M, Moraes LKA, Felisbino C, Crestani L, Guerra MP, Da Silva ALL (2003) Acclimatization of rootstocks Prunnus sp. micropropagated. Rev Bras Frut 25(2):279–281. doi:10.1590/S0100-29452003000200024
Sacchi GA, Morgutti S, Abruzzese A (1995) Changes in some physiological and biochemical parameters during two subcultures in kiwi (Actinidia deliciosa) callus. Plant Sci 106:107–113. doi:10.1016/0168-9452(94)04050-Q
Storck RC (2008) Sombreamento, ácido giberélico, e extrato de alga no desenvolvimento e produção de óleos essenciais em Patchouli. Dissertation, Universidade Federal do Paraná
Vandendool H, Kratz JDJ (1963) A generalization of the retention index system including linear temperature programmed gas-liquid partition chromatography. J Chromatogr 11:463–471
Verpoorte R, Contin A, Memelink J (2002) Biotechnology for the production of plant secondary metabolites. Phytochem Rev 1:13–25. doi:10.1023/A:1015871916833
Mohapatra H, Barik DP, Rath SP (2008) In vitro regeneration of medicinal plant Centella asiatica. Biol Plantarum 52(2):339–342. doi:10.1007/s10535-008-0069-5
Zhou X, Yang Han Y, Yang W, Xi T (1992) Somatic embryogenesis and analysis of peroxidase in cultured lettuce (Lactuca sativa L.) cotyledons. Ann Bot 69:97–100
Acknowledgments
We thank the FAPITEC/SE for funding this research and for granting a Master’s Scholarship to the first author. We thank CNPq for the research productivity grants awarded to the second and third author.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Santos, A.V., de Fátima Arrigoni-Blank, M., Blank, A.F. et al. Biochemical profile of callus cultures of Pogostemon cablin (Blanco) Benth. Plant Cell Tiss Organ Cult 107, 35–43 (2011). https://doi.org/10.1007/s11240-011-9953-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11240-011-9953-3