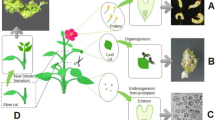
Abstract
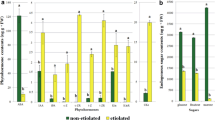
In an attempt to obtain insight into the differential responsiveness of different genotypes of Kalopanax septemlobus regarding their embryogenic capacity, several parameters such as endogenous hormonal levels, DNA content, embryogenic callus proliferation and somatic embryogenesis were studied in several genotypes of this plant. Also, to understand the effect of the age of the explants on their embryogenic capacity, the same parameters were studied in two embryogenic cell lines of different ages in the selected genotype. In the present study, it was observed that the cytokinins/abscisic acid (ABA) ratio plays an important role in embryogenic capacity in the studied genotypes of K. septemlobus species. A decrease in embryogenic capacity of callus was observed with increasing age, along with a marginal decrease in the DNA content of nuclei. Further, it can be suggested from our results that young embryogenic callus is a better choice for somatic embryo formation than the long-term-maintained callus in K. septemlobus.

Similar content being viewed by others
References
Abe T, Ii N, Togashi A, Sasahara T (2002) Large deletions in chloroplast DNA of rice calli after long-term culture. J Plant Physiol 159:917–923
Bajaj S, Rajam MV (1996) Polyamine accumulation and near loss of morphogenesis in long-term callus cultures of rice: restoration of plant regeneration by manipulation of cellular polyamine levels. Plant Physiol 112:1343–1348
Breton D, Harvengt L, Trontin JF, Bouvet A, Favre JM (2005) High subculture frequency, maltose-based and hormone-free medium sustained early development of somatic embryos in Maritime pine. In Vitro Cell Dev Biol Plant 41:494–504
Centeno ML, Rodríguez R, Berros B, Rodríguez A (1997) Endogenous hormonal content and somatic embryogenic capacity of Corylus avellana L. cotyledons. Plant Cell Rep 17:139–144
Choi JW, Huh K, Kim SH, Lee KT, Park HJ, Han YN (2002) Actinociceptive and anti-rheumatoidal effects of Kalopanax pictus extract and its saponin components in experimental animals. J Ethnopharmacol 79:199–204
Cvikrová M, Meravý L, Macháčková I, Eder J (1991) Phenylalanine ammonia-lyase, phenolic acids and ethylene in alfalfa (Medicago sativa L.) cell cultures in relation to their embryogenic ability. Plant Cell Rep 10:251–255
Etienne H, Sotta B, Montoro P, Miginiac E, Carron M-P (1993) Relations between exogenous growth regulators and endogenous indole-3-acetic acid and abscisic acid in the expression of somatic embryogenesis in Hevea brasiliensis (Müll. Arg.). Plant Sci 88:91–96
Fernández B, Centeno ML, Feito I, Sánchez-Tamés R, Rodríguez A (1995) Simultaneous analysis of cytokinins, auxins and abscisic acid by combined immunoaffinity chromatography, high performance liquid chromatography and immunoassay. Phytochem Anal 6:49–54
Hao YJ, Deng XX (2002) Occurrence of chromosomal variations and plant regeneration from long-term-cultured citrus callus. In Vitro Cell Dev Biol Plant 38:472–476
Ivanova A, Velcheva M, Denchev P, Atanassov A, van Onckelen A (1994) Endogenous hormone levels during direct somatic embryogenesis in Medicago falcata. Physiol Plant 92:85–89
Johnston JS, Bennett MD, Rayburn AL, Galbraith DW, Price HJ (1999) Reference standards for determination of DNA content of plant nuclei. Am J Bot 86:609–613
Klimaszewska K, Park YS, Overton C, Maceacheron I, Bonga JM (2001) Optimized somatic embryogenesis in Pinus strobus L. In Vitro Cell Dev Biol Plant 37:392–399
Klimaszewska K, Noceda C, Pelletier G, Label P, Rodríguez R, Lelu-Walter MA (2009) Biological characterization of young and aged embryogenic cultures of Pinus pinaster (Ait.). In Vitro Cell Dev Biol Plant 45:20–33
Lee EB, Li DW, Hyun JE, Kim IH, Whang WK (2001) Anti-inflammatory activity of methanol extract of Kalopanax pictus bark and its fractions. J Ethnopharmacol 77:197–201
Lelu-Walter MA, Bernier-Cardou M, Klimaszewska K (2006) Simplified and improved somatic embryogenesis for clonal propagation of Pinus pinaster (Ait.). Plant Cell Rep 25:767–776
Moon HK, Kim YW, Lee JS, Choi YE (2005) Micropropagation of Kalopanax pictus tree via somatic embryogenesis. In Vitro Cell Dev Biol Plant 41:303–306
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15:473–497
Nickle TC, Yeung EC (1994) Further evidence of a role for abscisic acid in conversion of somatic embryos of Daucus carota. In Vitro Cell Dev Biol Plant 30:96–103
Oropeza M, Marcano AK, García ED (2001) Proteins related with embryogenic potential in callus and cell suspensions of sugarcane (Saccharum sp.). In Vitro Cell Dev Biol Plant 37:211–216
Park HJ, Kim DH, Choi JW, Park JH, Han YN (1998) A potent anti-diabetic agent from Kalopanax pictus. Arch Pharm Res 21:24–29
Rajasekaran K, Hein MB, Vasil IK (1987) Endogenous abscisic acid and indole-3-acetic acid and somatic embryogenesis in cultured leaf explants of Pennisetum purpureum Schum.: effects in vivo and in vitro of glyphosate, fluridone, and paclobutrazol. Plant Physiol 84:47–51
Rodríguez APM, Wetzstein HY (1994) The effect of auxin type and concentration on pecan (Carya illinoinensis) somatic embryo morphology and subsequent conversion into plants. Plant Cell Rep 13:607–611
Shin JS, Kim YM, Hong SS, Kang HS, Yang YJ, Lee DK, Hwang BY, Ro JS, Lee MK (2005) Induction of neurite outgrowth by (-)-(7R, 8S)-dihydrodehyd-rodiconiferyl alcohol from PC12 Cells. Arch Pharm Res 28:1337–1340
Valdés AE, Centeno ML, Espinel S, Fernández B (2002) Could plant hormones be the basis of maturation indices in Pinus radiata? Plant Physiol Biochem 40:211–216
Williams EG, Maheswaran G (1986) Somatic embryogenesis: factors influencing coordinated behaviour of cells as an embryogenic group. Ann Bot 57:443–462
Yeoung YR, Lee MH, Kim BS, Kim HK, Kim JH (2001) Seed germination and softwood cutting technique of Kanlopanax pictus Nakai. Kor J Plant Res 14:53–59
Yeung EC (1999) The use of histology in the study of plant tissue culture systems—some practical comments. In Vitro Cell Dev Biol Plant 35:137–143
Zheng Q, Dessai AP, Prakash CS (1996) Rapid and repetitive plant regeneration in sweet potato via somatic embryogenesis. Plant Cell Rep 15:381–385
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Park, SY., Cho, HM., Moon, HK. et al. Genotypic variation and aging effects on the embryogenic capability of Kalopanax septemlobus . Plant Cell Tiss Organ Cult 105, 265–270 (2011). https://doi.org/10.1007/s11240-010-9862-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11240-010-9862-x