Abstract
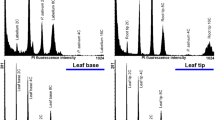
In this study, flow cytometric analysis was used to evaluate the genetic stability of Passiflora cincinnata Mast. plants regenerated via primary and secondary somatic embryogenesis. Embryogenic calli obtained from culturing zygotic embryos on Murashige and Skoog (MS) medium containing 18.1 μM 2,4-dichlorophenoxyacetic acid (2,4-D) and 4.4 μM benzyladenine (BA) were transferred to differentiation medium. Torpedo and cotyledonary embryos were obtained. These primary embryos were maintained on differentiation medium to generate secondary embryos. Conversion of primary and secondary embryos yielded 305 and 138 normal plants, respectively. Almost 90% of plantlets survived following acclimatization. Flow cytometric analysis revealed that seed-derived plants had on average 3.01 pg nuclear DNA (2C), and all plants, except for a single plant regenerated via primary embryogenesis, maintained their ploidy. This single plant contained more than twice the average DNA content: 6.21 pg (4C). Epidermal stomata of leaves of the tetraploid plant were larger but lower in density than those of diploid plants, indicating that stomatal characteristics are useful in distinguishing between diploid and tetraploid plants of passion fruit. In summary, the procedure we employed to regenerated P. cincinnata plants via somatic embryogenesis generated mostly genetically true-to-type plants.




Similar content being viewed by others
References
Alan AR, Zeng H, Assani A, Shi WI, Mcrae HE, Murch SJ, Saxena PK (2007) Assessment of genetic stability of the germplasm lines of medicinal plant Scutellaria baicalensis Georgi (Huang-qin) in long-term, in vitro maintained cultures. Plant Cell Rep 26:1345–1355. doi:10.1007/s00299-007-0332-9
Barbosa LV, Mondin M, Oliveira CA, Souza AP, Vieira MLC (2007) Cytological behaviour of the somatic hybrids Passiflora edulis f. flavicarpa + P. cincinnata. Plant Breed 126:323–328. doi:10.1111/j.1439-0523.2007.01362.x
Beck SL, Dunlop RW, Fossey A (2003) Stomatal length and frequency as a measure of ploidy level in black wattle, Acacia mearnsii (de Wild). Bot J Linn Soc 141:177–181. doi:10.1046/j.1095-8339.2003.00132.x
Bennet MD, Bhandol P, Leitch IJ (2000) Nuclear DNA amounts in angiosperms and their modern uses—807 new estimates. Ann Bot 86:859–909. doi:10.1006/anbo.2000.1253
Campos JMS, Davide LC, Salgado CC, Santos FC, Costa PN, Silva PS, Alves CCS, Viccini LF, Pereira AV (2009) In vitro induction of hexaploid plants from triploid hybrids of Pennisetum purpureum and Pennisetum glaucum. Plant Breed 128:101–104. doi:10.1111/j.1439-0523.2008.01546.x
Carvalho JFP, Carvalho CR, Otoni WC (2005) In vitro induction of polyploidy in annatto (Bixa orellana L.). Plant Cell Tiss Organ Cult 80:69–75. doi:10.1007/s11240-004-8833-5
Clarindo WR, Carvalho CR, Araújo FS, Abreu IS, Otoni WC (2008) Recovering polyploid papaya in vitro regenerants as screened by flow cytometry. Plant Cell Tiss Organ Cult 92:207–214. doi:10.1007/s11240-007-9325-1
Cuco SM, Vieira MLC, Mondin M, Aguiar-Perecin MLR (2005) Comparative karyotype analysis of three Passiflora L. species and cytogenetic characterization of somatic hybrids. Caryologia 58:220–228
De Verno LL (1995) An evaluation of somaclonal variation during somatic embryogenesis. In: Jain SM, Gupta PK, Newton RJ (eds) Somatic embryogenesis in woody plants. Kluwer Academic Publishers, Dordrecht, pp 361–377
de Vries SC, Booij H, Meyerink P, Huisman G, Wilde DH, Thomas TL, van Kammen A (1988) Acquisition of embryogenic potential in carrot cell-suspension culture. Planta 176:196–204. doi:10.1007/BF00392445
Doležel J, Bartos J (2005) Plant DNA flow cytometry and estimation of nuclear genome size. Ann Bot 95:99–110. doi:10.1093/aob/mci005
Doležel J, Lucretti S, Schubert I (1994) Plant chromosome analysis and sorting by flow cytometry. Crit Rev Plant Sci 13:275–309. doi:10.1080/713608061
Dornelas MC (1995) Culture and protoplasts fusion in Passiflora spp. Dissertation, Escola superior de agricultura luiz de queiroz (in portuguese)
Dudits D, Bogre L, Gyorgyey J (1991) Molecular and cellular approaches to the analysis of plant embryo development from somatic cells in vitro. J Cell Sci 99:475–484
Dudits D, Györgyey J, Bögre L, Bakó L (1995) Molecular biology of somatic embryogenesis. In: Thorpe TA (ed) In vitro embryogenesis in plants. Kluwer, Dordrecht, pp 267–308
Dunstan DI, Tautorus TE, Thorpe TA (1995) Somatic embryogenesis in woody plants. In: Thorpe TA (ed) In vitro embryogenesis in plants. Kluwer, Dordrecht, pp 471–538
Endemann M, Hristoforoglu K, Stauber T, Wilhelm E (2001) Assessment of age-related polyploidy in Quercus robur L. somatic embryos and regenerated plants using DNA flow cytometry. Biol Plant 44:339–345. doi:10.1023/A:1012426306493
Evans DA, Sharp WR, Medina-Filho HP (1984) Somaclonal and gametoclonal variation. Am J Bot 77:759–774
Fehér A (2005) Why somatic plant cells start to form embryos? In: Mujib A, Šamaj J (eds) Somatic embryogenesis. Springer Verlag, Berlin, pp 85–101. doi:10.1007/7089_019
Fehér A, Pasternak T, Ötvös K, Miskolczi P, Dudits D (2002) Induction of embryogenic competence in somatic plant cells: a review. Biologia 57:5–12
Fehér A, Pasternak T, Dudits D (2003) Transition of somatic plant cells to an embryogenic state. Plant Cell Tiss Organ Cult 74:201–228. doi:10.1023/A:1024072913021
Ferreira FR (2005) Passiflora genetics resources. In: Faleiro FG, Junqueira NTV, Braga MF (eds) Passionfruit: germplasm and breeding. Embrapa Cerrados, Planaltina, DF, Brazil, pp 40–52 (in Portuguese)
Filonova L, Bozhkov P, von Arnold S (2000) Developmental pathway of somatic embryogenesis in Picea abies as revealed by time-lapse tracking. J Exp Bot 51:249–264. doi:10.1093/jexbot/51.343.249
Fiuk A, Rybczyński JJ (2008) Genotype and plant growth regulator-dependent response of somatic embryogenesis from Gentiana spp. leaf explants In Vitro Cell. Dev Biol Plant 44:90–99. doi:10.1007/s11627-008-9124-3
Gamborg OL, Miller RA, Ojima K (1968) Nutrient requirements of suspension cultures of soybean root cells. Exp Cell Res 50:151–158. doi:10.1016/0014-4827(68)90403-5
Gesteira AS, Otoni WC, Barros EG, Moreira MA (2002) RAPD-based detection of genomic instability in soybean plants derived from somatic embryogenesis. Plant Breeding 121:269–271. doi:10.1046/j.1439-0523.2002.00708.x
Guerra MP, Dal Vesco L, Ducroquet PHJ, Nodari RO, Reis MS (2001) Somatic embryogenesis in goiabeira serrana: genotype response, auxinic shock and synthetic seeds. Braz J Plant Physiol 13:117–128
ITI Tropicals (2008) Available via www.passionfritjuice.com. Accessed 27 Out 2008
Jain SM (2001) Tissue culture-derived variation in crop improvement. Euphytica 118:153–166. doi:10.1023/A:1004124519479
Jain SM, De Klerk GJ (1998) Somaclonal variation in breeding and propagation of ornamental crops. Plant Tissue Cult Biotechnol 4:63–75
Jiménez VM, Thomas C (2005) Participation of plant hormones in determination and progression of somatic embryogenesis. In: Mujib A, Šamaj J (eds) somatic embryogenesis. Springer Verlag, Berlin, pp 103–118. doi:10.1007/7089_034
Kaparakis G, Alderson PG (2008) Role for cytokinins in somatic embryogenesis of pepper (Capsicum annuum L.). J Plant Growth Regul 27:110–114. doi:10.1007/s00344-007-9037-0
Karp A (1991) On the current understanding of somaclonal variation. Oxf Surv Plant Mol Cell Biol 7:1–58
Kim SW, Oh SC, Liu JR (2003) Control of direct and indirect somatic embryogenesis by exogenous growth regulators in immature zygotic embryo cultures of rose. Plant Cell Tiss Organ Cult 74:61–66. doi:10.1023/A:1023355729046
Larkin PJ, Scowcroft WR (1981) Somaclonal variation: a novel source of variability from cell cultures for plant improvement. Theor Appl Genet 60:197–214
Liu Z, Gao S (2007) Micropropagation and induction of autotetraploid plants of Chrysanthemum cinerariifolium (Trev.) Vis. In Vitro Cell Dev Biol Plant 43:404–408. doi:10.1007/s11627-007-9085-y
Liu G, Li Z, Bao M (2007) Colchicine-induced chromosome doubling in Platanus acerifolia and its effect on plant morphology. Euphytica 157:145–154. doi:10.1007/s10681-007-9406-6
Loureiro J, Pinto G, Lopes T, Doležel J, Santos C (2005) Assessment of ploidy stability of the somatic embryogenesis process in Quercus suber L. using flow cytometry. Planta 221:815–822. doi:10.1007/s00425-005-1492-x
May RA, Sink KC (1995) Genotype and auxin influence direct somatic embryogenesis from protoplasts derived from embryogenic cell suspension of Asparagus officinalis. Plant Sci 108:71–84. doi:10.1016/0168-9452(95)04117-D
Meletti LMM, Maia ML (1999) Passion fruit: production and commercialization. Technical Bulletim, Instituto Agronômico Estado de São Paulo, Campinas 181:1–62 (in Portuguese)
Meletti LMM, Soares-Scott MD, Bernacci LC, da S Passos IR (2005) Passion fruit breeding: past and future. In: Faleiro FG, Junqueira NTV, Braga MF (eds) Passion fruit: germplasm and breeding. Embrapa Cerrados, Planaltina, DF, Brazil, pp 55–78 (in Portuguese)
Motoike SY, Skirvin RM, Norton MA, Otterbacher AG (2001) Somatic embryogenesis and long term maintenance of embryogenic lines from fox grapes. Plant Cell Tiss Organ Cult 66:121–131
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15:473–497. doi:10.1111/j.1399-3054.1962.tb08052.x
Nomura K, Komamine A (1985) Identification and isolation of single cells that produce somatic embryos at a high frequency in a carrot suspension culture. Plant Physiol 79:988–991
Oliveira JC, Ruggiero C (2005) Passion fuit species with agronomic potential. In: Faleiro FG, Junqueira NTV, Braga MF (eds) Passionfruit: germplasm and breeding. Embrapa Cerrados, Planaltina, DF, Brazil, pp 142–158 (in Portuguese)
Orbovié V, Calovié M, Viloria Z, Nielsen B, Gmitter FG Jr, castle WS, Grosser JW (2008) Analysis of genetic variability in various tissue culture-derived lemon plant populations using RAPD and flow cytometry. Euphytica 161:329–335
Otoni WC, Blackhall NW, D’utra Vaz FB, Casali VWD, Power JB, Davey MR (1995) Somatic hybridization of the Passiflora species, P. edulis f. flavicarpa Degener and P. incarnata L. J Exp Bot 46:777–785
Paiva Neto VB, Botelho MN, Aguiar R, Silva EAM, Otoni WC (2003) Somatic embryogenesis from immature zygotic embryos of Annatto (Bixa orellana L.). In Vitro Cell Dev Biol Plant 39:629–634. doi:10.1079/IVP2003465
Passos IRS, Bernacci LC (2005) Tissue culture applied to in vitro germplasm conservation and breeding of passionfruit (Passiflora spp.). In: Faleiro FG, Junqueira NTV, Braga MF (eds) Passionfruit: germplasm and breeding. Embrapa Cerrados, Planaltina, DF, Brazil, pp 360–384 (in Portuguese)
Perl A, Saad S, Sahar N, Holland D (1995) Establishment of long-term embryogenic cultures of seedless Vitis vinifera cultivars–a synergistic effect of auxins and the role of abscisic acid. Plant Sci 104:193–200
Pfosser M, Amon A, Lelley T, Heberle-Bors E (1995) Evaluation of sensitivity of flow cytometry in detecting aneuploidy in wheat using disomic and ditelosomic wheat-rye addition lines. Cytometry 21:387–393
Pinto G, Park YS, Silva S, Neves L, Araújo C, Santos C (2008) Factors affecting maintenance, proliferation, and germination of secondary somatic embryos of Eucalyptus globulus Labill. Plant Cell Tiss Organ Cult 95:69–78
Pinto-Sintra AL (2007) Establishment of embryogenic cultures and plant regeneration in the Portuguese cultivar ‘Touriga Nacional’ of Vitis vinifera L. Plant Cell Tiss Organ Cult 88:253–265
Raghavan V (2004) Role of 2, 4-dichlorophenoxyacetic acid (2, 4-D) in somatic embryogenesis on cultured zygotic embryos of Arabidopsis: cell expansion, cell cycling, and morphogenesis during continuous exposure of embryos to 2, 4-D. Am J Bot 91:1743–1756
Rakoczy-Trojanowska M (2002) The effects of growth regulators on somaclonal variation in rye (Secale cereale L.) and selection of somaclonal variants with increased agronomic traits. Cell Mol Biol Lett 7:1111–1120
Rauf S, Khan IA, Khan FA (2006) Colchicine-induced tetraploidy and changes in allele frequencies in colchicine-treated populations of diploids assessed with rapid markers in Gossypium arboreum L. Turk J Biol 30:93–100
Silva ML, Paim Pinto DL, Guerra MP, Floh EIS, Bruckner CH, Otoni WC (2009) A novel regeneration system for a wild passionfruit (Passiflora cincinnata Mast.) species based on somatic embryogenesis from mature zygotic embryos. Plant Cell Tiss Organ Cult 99:47–54. doi:10.1007/s11240-009-9574-2
Souza MM, Palomino G, Pereira TNS, Pereira MG, Viana AP (2004) Flow cytometric analysis of genome size variation in some Passiflora species. Hereditas 141:31–38
Steiner N, Vieira FN, Maldonado S, Guerra MP (2005) Carbon source affects morphogenesis and histodifferentiation of A. angustifolia embryogenic cultures. Braz Arch Biol Technol 48:895–903
Vieira MLC, Oliveira EJ, Matta FP, Pádua JG, Monteiro M (2005) Biotechnological methods applied to passionfruit breeding. In: Faleiro FG, Junqueira NTV, Braga MF (eds) Passionfruit: germplasm and breeding. Embrapa Cerrados, Planaltina, DF, Brazil, pp 411–453 (in Portuguese)
Winkelmann T, Sangwan RS, Schwenkel HG (1998) Flow cytometric analyses in embryogenic and non-embryogenic callus lines of Cyclamen persicum Mill.: relation between ploidy level and competence for somatic embryogenesis. Plant Cell Rep 17:400–404
Yang XM, Cao ZY, An LZ, Wang YM, Fang XW (2006) In vitro tetraploid induction via colchicine treatment from diploid somatic embryos in grapevine (Vitis vinifera L.). Euphytica 152:217–224
Yang XM, An LZ, Xiong YC, Zhang JP LIY, Xu SJ (2008) Somatic embryogenesis from immature zygotic embryos and monitoring the genetic fidelity of regenerated plants in grapevine. Biol Plant 52:209–214
Zerbini FM, Otoni WC, Vieira MLC (2008) Passionfruit. In: Kole C, Hall TC (eds) A compendium of transgenic crop plants, v.5, tropical and subtropical fruit and nuts, 1st edn. Wiley, Berlin, pp 213–234
Zimermann JL (1993) Somatic embryogenesis: a model for early development in higher plants. Plant Cell 5:1141–1423
Acknowledgments
The authors would like to thank Fundação de Amparo à Pesquisa do Estado de Minas Gerais - FAPEMIG (Research grant number 1,527/2005), CAPES, and CNPq for financial support, and Prof. Zinmay Renee Sung for valuable suggestions regarding the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pinto, D.L.P., de Almeida Barros, B., Viccini, L.F. et al. Ploidy stability of somatic embryogenesis-derived Passiflora cincinnata Mast. plants as assessed by flow cytometry. Plant Cell Tiss Organ Cult 103, 71–79 (2010). https://doi.org/10.1007/s11240-010-9756-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11240-010-9756-y