Abstract
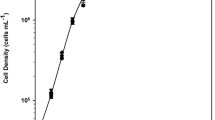
Gametophyte cells of brown algae Laminaria japonica were employed both in a modified silicone tubular membrane-aerated photobioreactor (bubble-less cultivation mode) and a bubble-column photobioreactor (bubbling cultivation mode), to study different gas–liquid mixing modes on cell growth rate and cell physiological status. With an inoculum density of 50 mg DCW l−1, in modified artificial Pacific seawater (APSW) medium at 13°C, light intensity of 60 μE m−2 s−1, light cycle of 16/8 h L/D, and aeration rate of 60 ml min−1, the specific growth rates were 0.082 d−1 for bubble-less mode and 0.070 d−1 for bubbling mode with biomass, in the form of dry cell density, increasing 10.9 and 6.8 times, respectively, during the 36 days’ photolithotrophic cultivation. The specific oxygen evolution rate under bubble-less mode was 39.6% higher than under bubbling mode on the 18th day. The gametophyte cells grew in cell aggregates with clump sizes, at day 36, of 1.5 mm and 0.5 mm diameter under bubble-less and bubbling mode respectively and cell injury percentages of 5.1% and 21.1%, respectively. The silicone tubular membrane-aerated photobioreactor was better suited for the cultivation of fragile macroalgal gametophyte cells due to the absence of hydrodynamic shear stress caused by fluid turbulence and the presence of a bubble-less gas supply.



Similar content being viewed by others
References
Abdussalam S (1990) Drugs from seaweeds. Med Hypotheses 32:33–35
Atkinson MJ, Smith SV (1983) C:N:P ratios of benthic marine plants. Limnol Oceanogr 28:568–574
Bohme C, Schroder MB, Jungheiliger H, Lehmann J (1997) Plant cell suspension culture in a bench-scale fermenter with a newly designed membrane stirred for bubble-free aeration. Appl Microbiol Biotechnol 48:148–154
Borowitzka MA, Larkum AWD (1976) Calcification in green alga-Halimeda. 2. Exchange of Ca2+ and occurrence of age gradients in calcification and photosynthesis. J Exp Bot 27:864–878
Brindle K, Stephenson T (1996) Application of membrane biological reactors for the treatment of wastewaters. Biotechnol Bioeng 49:601–610
Camacho FG, Grima EM, Miron AS, Pascual VG, Chisti Y (2001) Carboxymethyl cellulose protects algal cells against hydrodynamic stress. Enzyme Microb Technol 29:602–610
Camacho FG, Gomez AC, Sobczuk TM, Grima EM (2000) Effects of mechanical and hydrodynamic stress in agitated, sparged cultures of Porphyridium cruentum. Process Biochem 35:1045–1050
Carvalho AP, Malcata FX (2001) Transfer of carbon dioxide within cultures of microalgae: plain bubbling versus hollow-fiber modules. Biotechnol Prog 17:265–272
Casey E, Glennon B, Hamer G (1999) Oxygen mass transfer characteristics in a membrane-aerated biofilm reactor. J Eng Appl Sci 62:183–192
Chang CC, Tseng SK, Chang CC, Ho CM (2003) Reductive dechlorination of 2-chlorophenol in a hydrogenotrophic, gas-permeable, silicone membrane bioreactor. Bioresour Technol 90:323–328
Chung WC, Sun TH (1991) Continuous membrane fermentor separator for ethanol fermentation. J Memb Sci 57:21–42
Collos Y, Mornet F, Sciandra A (1999) An optical method for the rapid measurement of micromolar concentrations of nitrate in marine phytoplankton cultures. J Appl Phycol 11:179–184
Doig SD, Boam AT, Leak DI, Livingston AG, Stuckey DC (1998) Membrane bioreactor for biotransformations of hydrophobic molecules. Biotechnol Bioeng 58:587–594
Ducommun P, Ruffieux PA, Furter MP, Marison I, Stockar U (2000) A new method for on-line measurement of the volumetric oxygen uptake rate in membrane aerated animal cell cultures. J Biotechnol 78:139–147
Heussler P, Castillo J, Merino S, Vasquez V (1978) Improvement in pond construction and CO2 supply for the mass production of microalgae. Arch Hydrobiol 11:254–260
Huang YM, Rorrer GL (2002a) Dynamics of oxygen evolution and biomass production during cultivation of Agardhiella subulata microplantlets in a bubble-column photobioreactor under medium perfusion. Biotechnol Prog 18:62–71
Huang YM, Rorrer GL (2002b) Optimal temperature and photoperiod for the cultivation of Agardhiella subulata microplantlets in a bubble-column photobioreactor. Biotechnol Bioeng 79:135–144
Huang YM, Maliakal S, Cheney DP, Rorrer GL (1998) Comparison of development and photosynthetic growth for filament clumps and regenerated microplantlet cultures of Agardhiella subulata (Rhodophyta, Gigartinales). J Phycol 34:893–901
Hvoslef-Eide AK, Olsen OA, Lyngved R, Munster C, Heyerdahl PH (2005) Bioreactor design for propagation of somatic embryos. Plant Cell Tiss Org Cult 81:265–276
Jeffery SW, Humphrey GF (1975) New spectrophotometric equations for determining chlorophylls a, b, c1 and c2 in higher-plants, algae and natural phytoplankton. Biochem Physiol Pflanz 167:191–194
Joshi JB, Elias CB, Patole MS (1996) Role of hydrodynamic shear in the cultivation of animal, plant and microbial cells. Chem Eng J 62:121–141
Light BR, Beardall J (1998) Distribution and spatial variation of benthic microalgae biomass in a temperate, shallow-water marine system. Aquat Bot 61:39–54
Magaud F, Souhar M, Wild G, Boisson N (2001) Experimental study of bubble column hydrodynamics. Chem Eng Sci 56:4597–4607
Maksimova IV, Matorin DN, Plekhanov SE, Vladimirova MG, Volgin SL, Maslova IP (2000) Optimization of maintenance conditions for some microforms of red algae in collections. Russian J Plant Physiol 47:779–785
Malik KA (1995) A convenient method to maintain unicellular green algae for long times as standing liquid cultures. J Microbiol Methods 22:221–227
Martinez ME, Jimenez JM, Yousfi FE (1999) Influence of phosphorus concentration and temperature on growth and phosphorus uptake by the microalga Scenedesmus obliquus. Bioresour Technol 67:233–240
Miron AS, Garcia MCC, Gomez AC, Camacho FG, Grima EM, Chisti Y (2003) Shear stress tolerance and biochemical characterization of Phaeodactylum tricornutum in quasi steady-state continuous culture in outdoor photobioreactors. Biochem Eng J 16:287–297
Montaini E, Chini Zittelli G, Tredici MR, Molina Grima E, Fernandez Sevilla JM, Sanchez Perez JA (1995) Long-term preservation of Tetraselmis suecica: influence of storage on viability and fatty acid profile. Aquaculture 134:81–90
Papoutsakis ET (1991) Fluid-mechanical damage of animal cells in bioreactors. Trends Biotechnol 9:427–464
Pedersen MF (1994) Transient ammonium uptake in the macroalgal Ulva lactuca: nature regulation and the consequences for choice of measuring technique. J Phycol 30:980–986
Polzin JP, Rorrer GL (2003a) Halogenated monoterpene production by microplantlets of the marine red alga Ochtodes secundiramea within an airlift photobioreactor under nutrient medium perfusion. Biotechnol Bioeng 82:415–428
Polzin JP, Rorrer GL, Cheney DP (2003b) Metabolic flux analysis of halogenated monoterpene biosynthesis in microplantlets of the macrophytic red alga Ochtodes secundiramea. Biomol Eng 20:205–215
Qi H, Rorrer GL (1995) Photolithotrophic cultivation of Laminaria saccharina gametophyte cells in a stirred-tank bioreactor. Biotechnol Bioeng 45:251–260
Qi HN, Goudar CT, Michaels JD, Hans-Jugen H, Jovanovic GN, Konstantinov KB (2003) Experimental and theoretical analysis of tubular membrane aeration for mammalian cell bioreactors. Biotechnol Prog 19:1183–1189
Rai LC, Gaur JP (2001) Algal adaptation to environmental stresses: physiological, biochemical and molecular mechanisms. Springer-Verlag Publications, Heidelberg
Rausch C, Bucher M (2002) Molecular mechanisms of phosphate transport in plants. Planta 216:23–37
Rorrer GL, Cheney DP (2004) Bioprocess engineering of cell and tissue cultures for marine seaweeds. Aquacult Eng 32:11–41
Rorrer GL, Mullikin RK (1999) Modeling and simulation of a tubular recycle photobioreactor for macroalgal cell suspension cultures. Chem Eng Sci 54:3153–3162
Sajc L, Grubisic D, Vunjak-Novakovic G (2000) Bioreactors for plant engineering: an outlook for further research. Biochem Eng J 4:89–99
South GR, Whittick A (1987) Introduction to phycology. Blackwell Scientific Publications, UK
Ston J, Kosakowska A, Lotocka M (2002) Pigment composition in relation to phytoplankton community structure and nutrient content in the Baltic Sea. Oceanologia 44:419–437
Strickland JDH, Parsons TR (1968) A practical handbook of seawater analysis. Fisheries research board of Canada, Ottawa
Su WW, Caram HS, Humphrey AE (1992) Optimal design of the tubular microporous membrane aerator for shear-sensitive cell cultures. Biotechol Prog 8:19–24
Thomas TE, Harrison PJ (1985) Effects of nitrogen supply on nitrogen uptake, accumulation and assimilation in Porphyra perforate (Rhodophyta). Mar Biol 85:269–278
Tramper J, Smit D, Straatman J, Vlak JM (1987) Bubble-column design for growth of fragile insect cells. Bioprocess Eng 3:37–41
Zhang X, Li DP, Zhang YP, Zhang XY, Cai ZL, Wei C, Fan OY (2002) Comparison of photobioreactors for cultivation of Undaria pinnatifida gametophytes. Biotechnol Lett 24:1499–1503
Zhi C, Rorrer GL (1996) Photolithotrophic cultivation of Laminaria saccharina gametophyte cells in a bubble-column bioreactor. Enzyme Microb Technol 18:291–299
Zou N, Zhou B, Li B, Sun D, Zeng C (2003) Effects of cell density, light intensity and mixing on Undaria pinnatifida gametophyte activity in a photobioreactor. Biomol Eng 20:281–284
Acknowledgments
The authors gratefully acknowledge the High Technology Research and Development Project of China for supporting this research. The authors also thank Ocean Institute of Chinese Academy of Sciences for providing initial cell cultures of L. japonica gametophyte cells.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chen, S., Qi, H. Photolithotrophic cultivation of Laminaria japonica gametophyte cells in a silicone tubular membrane-aerated photobioreactor. Plant Cell Tiss Organ Cult 93, 29–38 (2008). https://doi.org/10.1007/s11240-008-9339-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11240-008-9339-3