Abstract
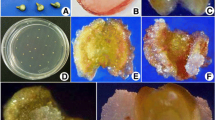
Conditions for induction of androgenesis in coconut cv. Sri Lanka Tall were studied. Anthers collected from inflorescences at four maturity stages were given heat (38°C) or cold (4°C) pretreatments for 1, 3, 6 and 14 days, either prior to or post inoculation. Three different basal media and different anther densities were also tested. Androgenesis was observed only in anthers collected from inflorescences 3 weeks before splitting (WBS) and after a heat pretreatment at 38°C for 6 days. Modified Eeuwens Y3 liquid medium supplemented with 100 μM 2,4-dichlorophenoxyacetic acid (2,4-d), 0.1% activated charcoal and 9% sucrose was effective in inducing an androgenic response. The lowest anther density tested, 10 per petri plate, was found to be the optimal density. When androgenic calli or embryos were subcultured to Y3 medium containing 66 μM 2,4-d, followed by transfer to Y3 medium without plant growth regulators and finally to Y3 medium containing 5 μM 6-benzyladenine (BA) and 0.35 μM gibberellic acid (GA3), plantlets regenerated at a frequency of 7%. Histological study indicated that the calli and embryos originated from the inner tissues of the anthers. Ploidy analysis of calli and embryos showed that they were haploid. This is the first report of successful androgenesis yielding haploid plants from coconut anthers.






Similar content being viewed by others
Abbreviations
- BA:
-
Benzyladenine
- CLZ:
-
Cambium like zone
- 2,4-d :
-
2,4-dichlorophenoxyacetic acid
- GA3 :
-
Gibberellic acid
- NBB:
-
Naphthol blue black
- PAS:
-
Periodic acid Schiff’s reaction
- WBS:
-
Weeks before splitting
References
Achar PN (2002) A study of factors affecting embryo yields from anther culture of cabbage. Plant Cell Tissue Organ Cult 69:183–188
Arnison PG, Donaldson P, Ho LCC, Keller WA (1990) The influence of various physical parameters on anther culture of broccoli (Brassica oleracea var. italica). Plant Cell Tissue Organ Cult 20:147–155
Ball ST, Zhou H, Konzak CF (1993) Influence of 2, 4-d, IAA and duration of callus induction in anther culture of spring wheat. Plant Sci 90:195–200
Buffard-Morel J, Verdeil JL, Pannetier C (1992) Embryogenèse somatique du cocotier (Cocos nucifera L.) à partir d’explants foliaires: Étude histologique. Can J Bot 70:735–741
Chatelet P, Gindreau K, Herve Y (1999) Development and use of microspore culture applied to vegetable Brassica oleracea breeding. In: Clement C, Pacini E, Audran J-C (eds) Anther and pollen. Springer-Verlag, Berlin, pp 247–259
Compton ME (1994) Statistical methods suitable for the analysis of plant tissue culture data. Plant Cell Tissue Organ Cult 37:217–242
Devaux O, Hou L, Ullrich E, Huang Z, Kleinhof A (1993) Factors affecting anther culturability of recalcitrant barley genotypes. Plant Cell Rep 13:32–36
Dolezel J, Binarova P, Lucretti S (1989) Analysis of nuclear DNA content in plant cells by flow cytometry. Biol Plant 31:113–120
Dunwell JM (1985) Anther and ovary culture. In: Bright SWJ, Jones MGK (eds) Cereal tissue and cell culture. Martinus Nijhoff/Dr W Junk Publishers, Dordrecht, Netherlands, pp 1–44
Ebert A, Taylor HF (1990) Assessment of the changes of 2,4-dichlorophenoxyacetic acid concentrations in plant tissue culture media in the presence of activated charcoal. Plant Cell Tissue Organ Cult 20:165–172
Fernando SC, Gamage CKA (2000) Abscisic acid induced somatic embryogenesis in immature embryo explants of coconut (Cocos nucifera L.). Plant Sci 151:193–198
Fernando SC, Verdeil JL, Hocher V, Weerakoon LK, Hirimburegama K (2003) Histological analysis of plant regeneration from plumule explants of Cocos nucifera L. Plant Cell Tissue Organ Cult 72:281–284
Fisher DB (1968) Protein staining of ribboned epon section for light microscopy. Histochemie 16:92–96
George EF (1993) Plant propagation by tissue culture. Part I, the technology. Exegetics Limited, Edington, England
Guo Y-D, Sewon P, Pulli S (1999) Improved embryogenesis from anther culture and plant regeneration in timothy. Plant Cell Tissue Organ Cult 57: 85–93
Hou L, Ullrich SE, Kleinhofs A, Stiff CM (1993) Improvement of anther culture methods for double haploid production in barley breeding. Plant Cell Rep 12:334–338
Immonen S, Anttila H (1999) Cold pretreatment to enhance green plant regeneration from rye anther culture. Plant Cell Tissue Organ Cult 57:121–127
Ishizaka H (1998) Production of microspore derived plants by anther culture of an inter-specific F1 hybrid between Cyclamen persicum × C. Purpurascens. Plant Cell Tissue Organ Cult 54:21–28
Iyer RD (1981) In: Rao AN (ed) Proceedings COSTED symposium on tissue culture of economically important plants. National University Singapore, Singapore, pp 219–230
Jacquard C, Asakaviciute R, Hamalian AM (2006) Barley anther culture: effects of annual cycle and spike position on microspore embryogenesis and albinism. Plant Cell Rep 25:375–381
Karunaratne S, Periyapperuma K (1989) Culture of immature embryos of coconut (Cocos nucifera L.): Callus proliferation and somatic embryogenesis. Plant Sci 62:247–253
Kernan Z, Ferrie AMR (2005) Microspore embryogenesis and the development of a double haploidy protocol for cow cockle (Saponaria vaccaria). Plant Cell Rep 25:274–280
Kim M, Kim J, Yoon M, Choi D, Lee M (2004) Origin of multicellular pollen and pollen embryos in cultured anthers of pepper (Capsicum annum). Plant Cell Tissue Organ Cult 77:63–72
Konieczny R, Czaplicki AZ, Golczyk H, Przywara L (2003) Two pathways of plant regeneration in wheat anther culture. Plant Cell Tissue Organ Cult 73:177–187
Kovoor A (1981) Palm tissue culture: state of art and its application to the coconut. FAO Plant production and protection paper 30. FAO, Rome
Metwally EI, Moustafa SA, Ei-Sawy BI, Shalaby TA (1998) Haploid plantlets derived by anther culture of Cucurbita pepo. Plant Cell Tissue Organ Cult 52: 171–176
Monfort S (1985) Androgenesis of coconut: embryos from anther culture. Z Pflanzenzuchtg 94:251–254
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15:473–497
Ochatt S, Zhang Y (1996) Haploid plants from pollen grains. Science 163: 85–87
Peixe A, Barroso J, Potes A, Pais MS (2004) Induction of haploid morphogenic calluses from in vitro cultured anthers of Prunus armeniaca cv. ‘Harcot’. Plant Cell Tissue Organ Cult 77:35–41
Peng M, Wolyn DJ (1999) Improved callus formation and plant regeneration for shed microspore culture in asparagus (Asparagus officinalis L.). Plant Cell Rep 18: 954–958
Perera PIP (2003) Cytological examination of pollen development for microspore and anther culture of coconut (Cocos nucifera L.) cv Sri Lanka Tall. Cocos 15:53–59
Perera PIP, Hocher V, Verdiil JL, Doulbeau S, Yakandawala DMD, Weerakoon LK (2007). Unfertilised ovary: a novel explant for coconut (Cocos nucifera L.) somatic embryogenesis. Plant Cell Rep 26: 21–28
Raina SK, Iyre RD (1983) Multicelled pollen pro-embryoids and callus formation in tea anther culture. J Plant Crops (Supplement):63–67
Reynolds TL (1997) Pollen embryogenesis. Plant Mol Biol 33:1–10
Rodrigues LR, Oliverira JMS, Mariath JEA, Iranco LB, Bodanese-Zanettini MH (2005) Anther culture and cold treatment of floral buds increased symmetrical and extra nuclei frequencies in soybean pollen grains. Plant Cell Tissue Organ Cult 81:101–104
Sandoval A, Hocher V, Verdeil JL (2003) Flow cytometric analysis of the cell cycle in different coconut palm (Cocos nucifera L.) tissues cultured in vitro. Plant Cell Rep 22:25–31
SAS Institute Inc. (1999) SAS/STAT user’s guide, version 7-1. SAS Institute Inc. Cary, North Carolina
Shimada T (1981) Haploid plants regenerated from the pollen callus of wheat (Triticum aestivum L.). Jpn J Genet 56:581–588
Thanh-Tuyen NT (1985) Anther culture: its prospects to coconut improvement. Philipp J Crop Sci 10:28–35
Thanh-Tuyen NT, De Guzman EV (1983) Formation of pollen embryos in cultured anthers of coconut (Cocos nucifera L.). Plant Sci Lett 29:81–88
Verdeil JL, Buffard-Morel J, Pannetier C (1989) Somatic embryogenesis of coconut (Cocos nucifera L.) from leaf and inflorescence tissue. Research findings and prospects. Oleagineux 44:403–411
Weerakoon LK (2004) Coconut tissue and embryo culture in Sri Lanka: current developments and future challenges. In: Peiris TSG, Ranasinghe CS (eds) Proc intern conf coconut res inst Sri Lanka—Part 1 (Review papers and guest presentations). CRI, Lunuwila, Sri Lanka, pp 41–61
Wong C-K, Ko S-W, Woo S-C (1983) Regeneration of rice plantlets on NaCl-stressed medium by anther culture. Bot Bull Acad Sinica 24: 59–64
Zhao F-C, Nilanthi D, Yang Y-S, Wu H (2006) Anther culture and haploid plant regeneration in purple coneflower (Echinacea purpurea L.). Plant Cell Tissue Organ Cult 86: 55–62
Zheng MY, Konzak CF (1999) Effect of 2,4-dichlorophenoxyacetic acid on callus induction and plant regeneration in anther culture of wheat (Triticum asetivum L.). Plant Cell Rep 19:69–73
Zheng MY (2003) Microspore culture in wheat (Triticum aestivum)—doubled haploid production via induced embryogenesis. Plant Cell Tissue Organ Cult 73: 213–230
Acknowledgements
Authors acknowledge Mr. C. Duperray (INSERM, Montpellier, France) for the technical assistance in flow-cytometry. We are also thankful to Mr. J. D. J. S. Kularatne for the assistance in statistical analysis. Our special thanks go to Ms. Shantha Ramanayake for reading the manuscript critically.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Perera, P.I.P., Hocher, V., Verdeil, JL. et al. Androgenic potential in coconut (Cocos nucifera L.). Plant Cell Tiss Organ Cult 92, 293–302 (2008). https://doi.org/10.1007/s11240-008-9337-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11240-008-9337-5