Abstract
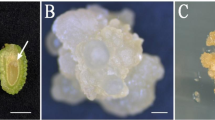
This is the first report describing culture conditions necessary to induce secondary embryogenesis in two carnation cultivars, Nelson and Spirit. In the first step, embryogenic calli were induced on petal explants followed by development of primary somatic embryos from the calli. In the second stage, secondary somatic embryos were obtained when precotyledonary and cotyledonary primary embryos were isolated and transferred onto a series of culture media all containing MS basal salt mixture, and supplemented with different concentrations of 2,4-D, BA, sucrose and mannitol. The highest rate of secondary embryogenesis occurred on mannitol containing media. Secondary somatic embryos were converted into plantlets when they were transferred onto growth regulator-free half-strength MS medium and successfully acclimated in the greenhouse.

Similar content being viewed by others
Abbreviations
- BA:
-
6-Benzyladenine
- 2,4-D:
-
2,4-Dichlorophenoxyacetic acid
- MS:
-
Murashige and Skoog basal medium
References
Agarwal S, Kanwar K, Sharma DR (2004) Factors affecting secondary somatic embryogenesis and embryo maturation in Morus alba L. Sci Hortic 102:359–368
Akula A, Becker D, Bateson M (2000) High-yielding repetitive somatic embryogenesis and plant recovery in a selected tea clone, ‘TRI-2025’, by temporary immersion. Plant Cell Rep 19:1140–1145
Ammirato PV (1987) Organizational events during somatic embryogenesis. In: Green CE, Somers DA, Hackett WP, Biesboer DD (eds) Plant tissue and cell culture. Alan R. Liss, New York, pp 57–81
Baker CM, Wetzstein HY (1995) Repetitive somatic embryogenesis in peanut cotyledon cultures by continual exposure to 2,4-D. Plant Cell Tissue Organ Cult 40:249–254
Burich GA, Mercun P, Benedtti L, Giovannini A (1996) Transformation method applicable to ornamental plant. Plant Tissue Cult Biotechnol 12:94–104
Carman JG (1990) Embryogenic cells in plant tissue cultures: occurrence and behavior. In vitro Cell Dev Biol Plant 26:746–753
Daigny G, Paul H, Sangwan RS, Sangwan-Narreel BS (1996) Factors influencing secondary somatic embryogenesis in Malus × domestica Borkh. (cv. ‘Gloster 69’). Plant Cell Rep 16:153–157
Eudes F, Acharya S, Laroche A, Selinger LB, Cheng KJ (2003) A novel method to induce direct somatic embryogenesis, secondary embryogenesis and regeneration of fertile green cereal plants. Plant Cell Tissue Organ Cult 73:147–157
Frey L, Saranga Y, Bjanik J (1992) Somatic embryogenesis in carnation. HortScience 27:63–65
Ghosh B, Sen S (1994) Plant regeneration from alginate encapsulated somatic embryos of Asparagus cooperi Baker. Plant Cell Rep 12:381–385
Ikeda-Iwai M, Umehara M, Satoh S, Kamada H (2002) Stress-induced somatic embryogenesis in vegetative tissue of Arabidopsis thaliana. Plant J 34:107–111
Kamada H, Ishikawa K, Saga H, Harada H (1993) Induction of somatic embryogenesis in carrot by osmotic stress. Plant Tissue Cult Lett 10:38–44
Laux T, Jurgens G (1997) Embryogenesis: new start in life. Plant Cell 9:989–1000
Little EL, Magbanua ZV, Parrot WA (2000) A protocol for repetitive somatic embryogenesis from mature peanut epicotyls. Plant Cell Rep 19:351–357
Lou H, Kako S (1995) Role of high sugar concentrations in inducing somatic embryogenesis from cucumber cotyledons. Sci Hortic 64:11–20
Maheswaran G, Williams EG (1984) Direct somatic embryoid formation on immature embryos of Trifolium repens, T. pratense and Medicago sativa, and rapid clonal propagation of T. repens. Ann Bot 54:201–221
Merkle SA, Parrott W, Flin BS (1995) Morphogenic aspect of somatic embryogenesis. In: Torpedoed in vitro embryogenesis in plant. Klauwer Academic Publishers, Dordrecht, pp 155–203
Nakano M, Mii M (1993) Antibiotic stimulates somatic embryogenesis without plant growth regulators in several dianthus cultivars. J Plant Physiol 14:721–725
Nakano M, Hoshio Y, Mii M (1994) Adventitious shoot regeneration from cultured petal expellant of carnation. Plant Cell Tissue Organ Cult 36:15–19
Neves LO, Duque SRL, Almeida JS, Fevereiro OS (1999) Repetitive somatic embryogenesis in Medicago trunculata ssp. Narborensis and M. trunculata Gaertn cv. Jemalong. Plant Cell Rep 18:398–405
Nontvaswatsri C, Fukai S, Touma T, Gol M (2002) Comparison of adventitious shoot formation from node and leaf explant of various carnation (Dianthus caryophyllus L.) cultivars. J Hortic Sci Biotechnol 77:520–525
Norgaard JV, Krogstrup P (1991) Cytokinin induced somatic embryogenesis from immature embryos of Abies nordmanniana LK. Plant Cell Rep 9:509–513
Nugent G, Ricnardoson T, Tandul CY (1991) Plant regeneration from stem and petal of carnation. Plant Cell Tissue Organ Cult 10:477–480
Raemakers CJJM, Jacobsen E, Visser RGF (1995) Secondary somatic embryogenesis and applications in plant breeding. Euphytica 81:93–107
Robert DR (1991) Abscisic asid and mannitol promote early development; maturation and storage protein accumulation in somatic embryogenesis of interior spruce. Plant Physiol 83:247–252
Sankhla D, Vavis TD, Shankla N, Upadya A (1995) In vitro of heat tolerant German carnation through organogenesis and somatic embryogenesis. Gartenbauwissenschaft 6:227–233
Vasic D, Alibert G, Skoric D (2002) Protocols for efficient and repetitive secondary somatic embryogenesis in Helliantus Maximiliani (Schrader). Plant Cell Rep 2:121–125
Williams EG, Maheswaran G (1986) Somatic embryogenesis: factors influencing coordinated behavior of cell as an embryogenic group. Ann Bot 57:443–462
Yantcheva A, Vlahova M, Antanassov A (1998) Direct somatic embryogenesis and plant regeneration of carnation (Dianthos caryophyllus L.). Plant Cell Rep 18:148–153
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Karami, O., Deljou, A. & Kordestani, G.K. Secondary somatic embryogenesis of carnation (Dianthus caryophyllus L.). Plant Cell Tiss Organ Cult 92, 273–280 (2008). https://doi.org/10.1007/s11240-007-9332-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11240-007-9332-2