Abstract
Since the beginning of the SARS-CoV-2 (COVID-19) pandemic, correlation of venous thromboembolism (VTE) and COVID-19 infection has been well established. Increased inflammatory response in the setting of COVID-19 infection is associated with VTE and hypercoagulability. Venous and arterial thrombotic events in COVID-19 infection have been well documented; however, few cases have been reported involving cardiac valve prostheses. In this review, we present a total of eight cases involving COVID-19-related prosthetic valve thrombosis (PVT), as identified in a systematic review. These eight cases describe valve position (mitral versus aortic) and prosthesis type (bioprosthetic versus mechanical), and all cases demonstrate incidents of PVT associated with simultaneous or recent COVID-19 infection. None of these eight cases display obvious non-adherence to anticoagulation; five of the cases occurred greater than three years after the most recent valve replacement. Our review offers insights into PVT in COVID-19 infected patients including an indication for increased monitoring in the peri-infectious period. We explore valve thrombosis as a mechanism for prosthetic valve failure. We describe potential differences in antithrombotic strategies that may offer added antithrombotic protection during COVID-19 infection. With the growing population of valve replacement patients and recurring COVID-19 infection surges, it is imperative to explore relationships between COVID-19 and PVT.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Highlights
-
COVID-19 related prosthetic valve failure has been reported in prosthetic valve patients of varying valve prosthesis types.
-
Prosthetic valve failure and valve thrombosis with COVID-19 infection has been reported in patients while on antithrombotic therapy.
-
With known hypercoagulability associated with COVID-19 infection, prosthetic valve failure and valve thrombosis is an important diagnosis to consider in COVID-19 infected patients.
-
Future study may better identify which patients may be at increased risk of prosthetic valve failure and valve thrombosis in the setting of COVID-19 infection.
Introduction
Since the beginning of the Sars-CoV-2 (COVID-19) pandemic, the relationship between infection and hypercoagulability has been well documented [1]. It is hypothesized that COVID-19 infected patients develop a hypercoagulable state as a result of increased inflammatory cytokine production and upregulation of coagulation pathways increasing the risk of developing thrombotic events [2,3,4]. While many of these COVID-19-related thrombotic events such as deep vein thrombosis (DVT), pulmonary embolism (PE), cerebrovascular accident (CVA), and myocardial infarction (MI) have been documented and reviewed [4], few have been reported as prosthetic valve thrombosis (PVT). Our goal is to collect and summarize all published cases of PVT associated with COVID-19 infection.
Methods
A systematic literature search in PubMed was conducting using the four search terms “COVID and Aortic Valve,” “COVID and Mitral Valve,” “COVID and Tricuspid Valve,” and “COVID and Pulmonic Valve” to identify PVT incidents in the setting of COVID-19 infection. Only articles that were published before July 14, 2022 were included. Each article was then reviewed and included if it was a case report deemed to be presenting PVT in the setting of COVID-19 infection. For each article, data on the following were gathered: the relation of time between valve replacement, COVID-19 infection, and PVT; the type (bioprosthetic vs mechanical) and position (mitral vs aortic) of the involved valve; prior anticoagulation/antiplatelet therapy; D-dimer levels; COVID-19 infection severity; history of other significant conditions; thrombus descriptions; imaging results; additional complications/consequences; treatments; and outcomes. Exclusion criteria included non-English publications, articles unrelated to prosthetic valve failure, articles about prosthetic valve failure outside the context of COVID-19 infection, and articles accounting COVID-19-related prosthetic valve endocarditis. Data was compiled using Excel.
Results
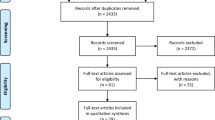
The initial literature review resulted in 268 journal articles. Of these, 244 were deemed unrelated to the focus of prosthetic valve failure, seven documented prosthetic valve failure outside the context of recent/simultaneous COVID-19 infection, and another four recorded COVID-19-related prosthetic valve endocarditis. Five were non-English publications. The remaining eight articles were all case studies and documented COVID-19-associated prosthetic valve thrombosis, the objective of this review (Fig. 1).
The eight COVID-19-related PVT cases documented in this paper include valve type (mitral versus aortic) and prosthesis type (bioprosthetic versus mechanical). Of these cases, five involved prostheses in the mitral valve position, three of which were mechanical [5,6,7] and two were bioprosthetic [8, 9]; three of the eight cases involved aortic valve prostheses, one being mechanical [10] and two being surgical bioprosthetic valves [11, 12] (Table 1). Seven of eight patients had symptoms of dyspnea or hypoxia [5,6,7,8,9, 11, 12]. Al Helali et al. presented a patient with a mechanical aortic valve prosthesis presenting with a change in character of cardiac auscultation and generalized fatigue [10].
In these eight total cases incident PVT was associated with simultaneous or recent COVID-19 infection. All patients in these cases tested positive for COVID-19 within two months prior to the PVT diagnosis; three PVT cases were diagnosed during hospitalization with COVID-19 [7, 10, 12]. Additionally, five cases occurred greater than three years after the most recent valve replacement [5,6,7,8, 10], and the other three bioprosthetic valves failed within eight months of valve replacement [9, 11, 12]. One case, described by Vinnakota et al., documented a recurrent case of PVT: 12 months after initial mitral and aortic valve replacements, the patient underwent transcatheter mitral valve-in-valve implantation for suspected early mitral bioprosthetic stenosis secondary to PVT, and seven months afterwards was diagnosed with COVID-19, presenting with a second PVT within the next month.
Seven of eight cases document patients having been on anticoagulation and/or antiplatelet therapy leading up to the PVT, for anti-thrombotic purposes relating to prostheses, atrial fibrillation (AF), and/or recent COVID-19 infection. Acenocoumarol, warfarin, apixaban, or aspirin were used, and none had documented medication non-adherence noted [5,6,7, 9,10,11,12].
Of the six cases that included, inflammatory markers were noted to be increased when included in the case reports – including elevated D-dimer [5, 7, 8, 10,11,12], ranging from 372 ng/mL [10] to 6893 ng/mL [5].
Five of eight cases described severe COVID-19 infection including hospitalization with associated respiratory distress and associated abnormal lung findings seen on imaging [5,6,7,8, 11]; one case further developed measured pulmonary hypertension and respiratory failure [5], one developed pulmonary edema subsequent to supraventricular tachycardia [7] and one developed pulmonary congestion with pleural effusion [8]. In addition to PVT, two of the eight cases documented other thrombotic events also presumed to be associated with COVID-19 infection: Manghat et al. diagnosed pulmonary embolism, embolic myocardial infarction, and symptoms of retinal artery embolism, and Llopis Gisbert et al. also included pulmonary embolism. Of all six cases with recorded D-dimer levels, these two cases described by Manghat et al. and Llopis Gisbert et al. displayed the second (5547 ng/mL) and third (3041 ng/mL) highest levels, respectively.
Two of the eight total cases document a history of chronic disease prior to the COVID-19-associated PVT. Vinnakota et al. describe a patient with end-stage renal failure and AF that developed PVT with an imaged in situ thrombus measuring 20 × 15 × 20 mm. Cardona Buitrago et al. describe a patient with permanent valvular AF, obesity, congenital single kidney, and hypothyroidism that developed two PVT events within eight months (as stated earlier), the latter being associated with COVID-19 infection and described as a “large thrombus” resulting in “near total occlusion of flow.”
In all eight cases, the COVID-19-associated PVT was diagnosed with echocardiography, computed tomography, and/or fluorography. Results showed thrombotic mass attached to the prosthesis (if specified, to leaflet(s), disc, or disc hinge point), reduced mobility of prosthetic leaflet(s), and/or thickened leaflet(s) (Fig. 2).
Collection of Images Presented with Selected Imaging Modalities. Top to Bottom: bioprosthetic aortic valve, mechanical aortic valve, mechanical mitral valve, bioprosthetic mitral valve. Left to Right Row 1 Alexander et al., TEE and CT images demonstrating leaflet thickening; Manghat et al., CT and MRI images visualizing paravalvular thrombus (arrows). Left to Right Row 2 Al Helali et al., Fluoroscopy and CT images demonstrating thrombus, pannus formation and restricted leaflet mobility. Left to Right Row 3 Jeckelman et al., TEE image demonstrating isoechoic mass; Cardona Buitrago et al., TEE image demonstrating hyperechogenic image; Aruğaslan et al., TEE image demonstrating restricted leaflet mobility and thrombus. Left to Right Row 4 Llopis Gisbert et al., TEE image demonstrating thrombus; Vinnakota et al., CT image demonstrating thrombus. TEE (Transesophageal Echocardiogram); CT (Computed Tomography); MRI (Magnetic Resonance Imaging) [5,6,7,8,9,10,11,12]
Half of the eight cases documented additional complications/consequences – all cardiac related – aside from the COVID-19-assocated respiratory difficulties or thrombotic events already described above. In the case reported by Jeckelmann et al., the patient had developed a new onset AF specifically related to the PVT. In the case described by Manghat et al., the patient’s PVT and embolic MI were also accompanied by pericardial effusion (possibly perioperative after recent replacement) and transient atrial flutter. In the case described by Aruğaslan et al., the patient experienced supraventricular tachycardia and subsequent pulmonary edema as mentioned before, alongside the re-elevation of mean pressure gradient amid ineffective anticoagulation treatment; after thrombolytics proved ineffective, emergency valve replacement was done. Lastly, in the case described by Cardona Buitrago et al., during recovery after emergency valve replacement, the patient experienced significant bradyarrhythmia with rhythm of escape from the junction, occasional premature ventricular complexes of two morphologies, pairs, bigeminy, and episodes of non-sustained ventricular tachycardia; antiarrhythmics and a unicameral pacemaker had to be administered.
Regarding treatment for the COVID-19-associated PVT (and other thrombotic events), three patients were treated only with anticoagulation as their most intensive form of therapy, using heparin and warfarin [11, 12] or heparin alone [8]. Another three patients were treated with thrombolytics as their most intensive therapy, using alteplase [5, 10] or tenecteplase [9]. Finally, two cases, those described by Cardona Buitrago et al. and Aruğaslan et al., ultimately required emergency valve replacement. In both of these cases, the replaced valves were mechanical mitral valves implanted more than 3 years prior, COVID-19 infection was confirmed within 20 days prior to PVT, and the patients were on warfarin therapy leading up to the incident.
All eight patients survived to hospital discharge. Follow-up imaging studies in all seven of the cases that documented them, done soon after or months after these therapies, revealed resolution of PVT, normal prosthesis function, and/or improvement of valve gradient [5, 6, 8,9,10,11,12].
Discussion
The hypercoagulable state associated with COVID-19 infection has been hypothesized to be related to host inflammatory response to the virus and associated increased inflammatory cytokine production [2,3,4]. Elbadawi et al. found that acute thrombotic events occurred in 5.2% of hospitalized patients with COVID-19; this 5.2% consisted of VTE (2.7%), ischemic stroke (1.2%), and MI (1.2%) [13]. Bilaoglu et al. reported an even higher incidence of thrombotic events among COVID-19 hospitalized patients (16.0%), with similar proportions [14]. Middeldorp et al. reported that of COVID-19-related VTE events, PE was the most frequent (81%) [15]. Based on our systematic review, the cases presented in this review are the only reported incidents of COVID-19-associated PVT. We support the authors’ proposed hypothesis that these eight incidents are at least partially a result of the pro-thrombotic nature of COVID-19 infection, as noted in this discussion.
It has been postulated that the severity of COVID-19 infection may correlate with the likelihood of subsequent thrombotic events, with the highest incidence among patients in the intensive care unit [16]. Consistent with these findings, most of these eight cases of PVT occurred in the context of severe COVID-19 infection, with patients being hospitalized for associated respiratory difficulties and displaying COVID-19 pneumonia lung lesions [5,6,7,8, 11].
The most correlative prognostic factor for severity/mortality of COVID-19 illness and associated thrombotic events is suggested to be elevated D-dimer [17,18,19,20]. Du et al. found a pooled odds ratio of 1.90 (95% CI: 1.32–2.48; P < 0.001) regarding the relationship between D-dimer and COVID-19, and Berger et al. found that patients with elevated D-dimer were more likely to have critical illness than those with normal D-dimer (43.9% versus 18.5%). Berger et al. also asserted that COVID-19 patients with elevated D-dimer were also more likely to undergo a thrombotic event (19.4% versus 10.2%). These findings are again consistent with the COVID-19-associated PVT cases presented in this review, with all six cases that included lab results displaying elevated D-dimer [5, 7, 8, 10,11,12]. Furthermore, Berger et al. found that patients with D-dimer > 2000 ng/mL had the highest risk of thrombotic event (37.8%), and it is interesting to note that four out of the six (66.7%) cases that included D-dimer levels in this review displayed values > 2000 ng/mL [5, 8, 11, 12]; perhaps an even higher D-dimer level–- and a more hypercoagulable state–- is associated with PVT compared to other thrombotic events associated with COVID-19 infection.
The general risk factors of PVT have been well described. Dürrleman et al. report that the mean time interval from first valve replacement to PVT was 39 ± 42 months [21], and it has been documented that PVT incidence is highest during the early post-operative period for both mechanical and bioprosthetic valves [22]. In comparison, PVT ranged from 2 weeks to 22–24 years) from first valve replacement in COVID-19-associated PVT for the eight cases in this review; five of these cases occurred greater than 3 years post-replacement [5,6,7,8, 10]. This wide range of time until PVT suggests COVID-19 infection might increase the risk of late PVT.
In general, mechanical valve PVT is generally more prevalent compared with bioprosthetic valve PVT [22, 23], with thrombosis incidence rate being 3–190 times greater for mechanical valves (0.1–5.7%) than for bioprosthetic valves (0.03%) [23]. For the eight cases in this review, the number of PVT incidents involving mechanical valves vs bioprosthetic valves were equal. This offers a possible hypothesis that COVID-19 infection may be associated with increased PVT risk in bioprosthetic valves. Mitral valve PVT is considered 2–3 times more frequent than aortic PVT [22], with 67% of all PVTs involving the mitral position and 15% involving the aortic position [21]. A roughly similar proportion was found in this review, with 5/8 (62.5%) involving mitral PVT and 3/8 (37.5%) involving aortic PVT; offering a hypothesis that COVID-19 infection may be associated with PVT independent of mitral or aortic valve position.
The most frequent risk factors contributing to PVT include inadequate anticoagulation, AF, recent infection, and plasma fibrinogen level, as reported by Bezanjani et al. Furthermore, with regards to inadequate anticoagulation, it has been recently recognized that public policy-related COVID-19 restriction measures have posed a challenge for patients and physicians to regularly assess patients’ INR levels and ensure effective anticoagulation; Vriz et al. found that the incidence of stuck prosthetic valves has significantly increased amid these restrictions [24]. While we acknowledge that COVID-19 restriction measures themselves may have had an influence on PVT incidence during the COVID-19 pandemic, it is important to note that none of the cases included in this review noted significant non-adherence to anticoagulation medication nor INR check-ups. This suggests that COVID-19 infection itself may increase the risk of PVT. In the context of the reported PVT cases presented, COVID-19 infection potentiated PVT and overcame the therapeutic effect of anticoagulation in those patients taking it. Thus, patients with prosthetic valves –both mechanical and bioprosthetic – and recently diagnosed with COVID-19 infection may warrant closer monitoring or more frequent therapeutic monitoring, and possibly higher doses of anticoagulants to potentially lower the risk of PVT. PVT is an important topic for future and ongoing investigation in better characterizing risks and risk groups of patients infected with COVID-19.
The patients included present heterogeneously and seemingly unpredictably. Limitations of this review include the inclusion criteria of English only publications and the inherent newness of COVID-19 and the evolving understanding of the various manifestations of interactions it may pose both short term and long term on human physiology and specifically valve prostheses. As COVID-19 continues to evolve and dynamically change and global prevalence continues, infection in the growing cohort of prosthetic valve patients with waning natural and vaccine related immunity or absent immune protection from emerging COVID-19 variants may further demonstrate clinical relevance of PVT in the setting of COVID-19 infection. Early diagnosis of PVT can modify treatment recommendations and improve the quality of care in patients with prosthetic valves.
Conclusions
With the known hypercoagulability associated with COVID-19 infection, prosthetic valve failure and valve thrombosis is an important diagnosis to consider in hospitalized COVID-19 infected patients.
Clinical implications
Prosthetic valve failure of the aortic and mitral valve positions thrombosis has been reported in patients with both mechanical and tissue valve prostheses with or without ongoing antithrombotic or antiplatelet therapy in the setting of COVID-19 infection. In patients with prosthetic valves, COVID-19 related valve failure is plausible to include as a consequence of infection.
Data availability
Not applicable.
Abbreviations
- PVT:
-
Prosthetic Valve Thrombosis
- COVID:
-
SARS-CoV-2
References
Birkeland K, Zimmer R, Kimchi A, Kedan I (2020) Venous thromboembolism in hospitalized COVID-19 patients: systematic review. Interact J Med Res 9(3):e22768. https://doi.org/10.2196/22768
Connors JM, Levy JH (2020) COVID-19 and its implications for thrombosis and anticoagulation. Blood 135(23):2033–2040. https://doi.org/10.1182/blood.2020006000
Hanff TC, Mohareb AM, Giri J, Cohen JB, Chirinos JA (2020) Thrombosis in COVID-19. Am J Hematol 95(12):1578–1589. https://doi.org/10.1002/ajh.25982
Abou-Ismail MY, Diamond A, Kapoor S, Arafah Y, Nayak L (2020) The hypercoagulable state in COVID-19: incidence, pathophysiology, and management. Thromb Res 194:101–115. https://doi.org/10.1016/j.thromres.2020.06.029
Jeckelmann C, Djokic B, Duchatelle V, Girod G (2022) Case report: mechanical mitral prosthetic valve thrombosis in the context of COVID-19 despite effective anticoagulation. Eur Heart J C Rep. https://doi.org/10.1093/ehjcr/ytac006
Cardona Buitrago C, Builes Gutierrez AM, Jiménez Marín D, Aristizábal García C (2022) Mechanical valve thrombosis secondary to severe acute respiratory syndrome coronavirus 2 infection: a case report. Cureus 14(3):e23358. https://doi.org/10.7759/cureus.23358
Aruğaslan E, Çalapkulu Y, Örnek E, Karanfil M, Bayram H, Küçüker SA (2022) Trombose mecânica da válvula Mitral em paciente com infecção por COVID-19. Arquivos brasileiros de cardiología 118(6):1141–1143. https://doi.org/10.36660/abc.20210581
Llopis Gisbert G, Vidal Urrutia V, Moruno Benita MA, Payá Chaume A, Berenguer Jofresa A, Cubillos Arango AM, Pérez Boscá JL, Payá Serrano R (2021) Bioprosthetic valve thrombosis and obstruction secondary to COVID-19. Can J Cardiol 37(6):938.e3-938.e6. https://doi.org/10.1016/j.cjca.2020.10.008
Vinnakota S, Jentzer JC, Luis SA (2021) Thrombolysis for COVID-19-associated bioprosthetic mitral valve thrombosis with shock. Eur Heart J 42(39):4093. https://doi.org/10.1093/eurheartj/ehab333
Al Helali S, Sandokji H, Al Moughari A, Al Ghamdi H, Assiri T, Al Amri H (2022) Successful use of ultraslow thrombolytic therapy in stuck mechanical aortic valve in a patient with COVID-19; a case report. Int J Surg Case Rep 95:107233. https://doi.org/10.1016/j.ijscr.2022.107233
Alexander SA, Fergus IV, Lerakis S (2021) Bioprosthetic valve thrombosis associated with COVID-19 Infection. Circ Cardiovasc Imagin 14(5):e012118. https://doi.org/10.1161/CIRCIMAGING.120.012118
Manghat NE, Hamilton M, Joshi NV, Vohra HA (2020) Acute postoperative thrombosis of an aortic valve prosthesis and embolic myocardial infarction in a coronavirus disease 2019 (COVID-19)-positive patient-an unrecognized complication. JTCVS Tech 4:111–113. https://doi.org/10.1016/j.xjtc.2020.09.020
Elbadawi A, Elgendy IY, Sahai A, Bhandari R, McCarthy M, Gomes M, Bishop GJ, Bartholomew JR, Kapadia S, Cameron SJ (2021) Incidence and outcomes of thrombotic events in symptomatic patients with COVID-19. Arterioscler Thromb Vasc Biol 41(1):545–547. https://doi.org/10.1161/ATVBAHA.120.315304
Bilaloglu S, Aphinyanaphongs Y, Jones S, Iturrate E, Hochman J, Berger JS (2020) Thrombosis in hospitalized patients with COVID-19 in a New York city health system. JAMA 324(8):799–801. https://doi.org/10.1001/jama.2020.13372
Middeldorp S, Coppens M, van Haaps TF, Foppen M, Vlaar AP, Müller M, Bouman C, Beenen L, Kootte RS, Heijmans J, Smits LP, Bonta PI, van Es N (2020) Incidence of venous thromboembolism in hospitalized patients with COVID-19. J Thromb haemost: JTH 18(8):1995–2002. https://doi.org/10.1111/jth.14888
Al-Ani F, Chehade S, Lazo-Langner A (2020) Thrombosis risk associated with COVID-19 infection a scoping review. Thromb Res 192:152–160. https://doi.org/10.1016/j.thromres.2020.05.039
Du WN, Zhang Y, Yu Y, Zhang RM (2021) D-dimer levels is associated with severe COVID-19 infections: a meta-analysis. Int J Clin Pract 75(8):e14031. https://doi.org/10.1111/ijcp.14031
Rostami M, Mansouritorghabeh H (2020) D-dimer level in COVID-19 infection: a systematic review. Expert Rev Hematol 13(11):1265–1275. https://doi.org/10.1080/17474086.2020.1831383
Zhan H, Chen H, Liu C, Cheng L, Yan S, Li H, Li Y (2021) Diagnostic value of D-dimer in COVID-19: a meta-analysis and meta-regression. Clin Appl Thromb/hemost. https://doi.org/10.1177/10760296211010976
Berger JS, Kunichoff D, Adhikari S, Ahuja T, Amoroso N, Aphinyanaphongs Y, Cao M, Goldenberg R, Hindenburg A, Horowitz J, Parnia S, Petrilli C, Reynolds H, Simon E, Slater J, Yaghi S, Yuriditsky E, Hochman J, Horwitz LI (2020) Prevalence and outcomes of D-dimer elevation in hospitalized patients with COVID-19. Arterioscler Thromb Vasc Biol 40(10):2539–2547. https://doi.org/10.1161/ATVBAHA.120.314872
Dürrleman N, Pellerin M, Bouchard D, Hébert Y, Cartier R, Perrault LP, Basmadjian A, Carrier M (2004) Prosthetic valve thrombosis: twenty-year experience at the montreal heart institute. J Thorac Cardiovasc Surg 127(5):1388–1392. https://doi.org/10.1016/j.jtcvs.2003.12.013
Roudaut R, Serri K, Lafitte S (2007) Thrombosis of prosthetic heart valves: diagnosis and therapeutic considerations. Heart (British Cardiac Soc) 93(1):137–142. https://doi.org/10.1136/hrt.2005.071183
Noohi Bezanjani F, Gohari S, Bassiri HA, Ahangar H, Reshadmanesh T (2020) risk factors associated with heart valve thrombosis in patients with prosthetic heart valve dysfunction. Arch Iran Med 23(9):600–604. https://doi.org/10.34172/aim.2020.70
Vriz O, Rossi Zadra A, Eltayeb A, Asiri F, Pragliola C, Fawzy N, Galzerano D, Feras K, Alhalees Z, Kinsara AJ, Elmula F (2021) Loss of engagement in controlling chronic anticoagulation therapy during Covid-19 stringency measures a single center experience of disproportioned increase of stuck mechanical valves. Monaldi Arch Ch Dis. https://doi.org/10.4081/monaldi.2021.2065
Acknowledgements
Not applicable
Funding
No funding was received for this review.
Author information
Authors and Affiliations
Contributions
TT researched, wrote, revised and had final determination over the manuscript submission. KB researched, wrote, revised and had final determination over the manuscript submission. AK assist with conception of topic and identified participating writing team members, reviewed and had final determination over manuscript. IK conceived of the topic, researched, wrote, revised and had final determination over the manuscript submission.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
Not applicable.
Consent for publication
Authors are responsible for correctness of the statements provided in the manuscript. The Editor-in-Chief reserves the right to reject submissions that do not meet the guidelines described in this section. Images will be removed from publication if authors have not obtained informed consent or the paper may be removed and replaced with a notice explaining the reason for removal.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Trieu, T.K., Birkeland, K., Kimchi, A. et al. Comprehensive collection of COVID-19 related prosthetic valve failure: a systematic review. J Thromb Thrombolysis 55, 474–489 (2023). https://doi.org/10.1007/s11239-022-02746-x
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11239-022-02746-x