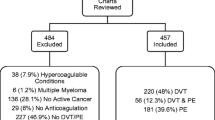
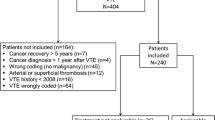
Abstract
Cancer associated thrombosis (CAT) is a leading cause of death among patients with cancer. It is not clear if non-clinical factors are associated with anticoagulation receipt. We conducted a retrospective cohort study of Optum’s de-identified Clinformatics® Database of adults with cancer diagnosed between 2009 and 2016 who developed CAT, treated with an outpatient anticoagulant (warfarin, low molecular weight heparin (LMWH), or a direct oral anticoagulant (DOAC)). Of 12,622 patients, three months after an episode of CAT, 1,485 (12%) were on LMWH, 1,546 (12%) on DOACs, and 9,591 (76%) were on warfarin. When controlling for other factors, anticoagulant use was significantly associated with socioeconomic factors, region, co-morbidities, type of thrombosis, and cancer subtype. Patients with a bachelor’s degree or greater level of education were less likely to receive warfarin (OR: 0.77; 95% CI: [0.59, 0.99]; p < 0.046) or DOACs (OR: 0.67; 95% CI: [0.55, 0.82]; p < 0.001) compared to LMWH. Patients with higher income levels were more likely to receive LMWH or DOACs compared to warfarin, while patients across all income levels were equally likely to receive LMWH or DOACs. Non-clinical factors including income, education, and region, are associated with anticoagulation receipt three months after an episode of CAT. Sociodemographic factors may result in some patients receiving suboptimal care and contribute to non-guideline concordant care for CAT.



Similar content being viewed by others
References
Hisada Y, Geddings JE, Ay C, Mackman N (2015) Venous thrombosis and cancer: from mouse models to clinical trials. J Thromb Haemost 13(8):1372–1382
Meyer G, Marjanovic Z, Valcke J et al (2002) Comparison of low-molecular-weight heparin and warfarin for the secondary prevention of venous thromboembolism in patients with cancer: a randomized controlled study. Arch Intern Med 162(15):1729–1735
Lee AYY, Peterso Lee AYY, Levine MN et al (2003) Low-molecular-weight heparin versus a coumarin for the prevention of recurrent venous thromboembolism in patients with cancer. N Engl J Med 349(2):146–153
Hull RD, Pineo GF, Brant RF et al (2006) Long-term low-molecular-weight heparin versus usual care in proximal-vein thrombosis patients with cancer. Am J Med 119(12):1062–1072
Lee AYY, Kamphuisen PW, Meyer G et al (2015) Tinzaparin vs warfarin for treatment of acute venous thromboembolism in patients with active cancer: a randomized clinical trial. JAMA 314(7):677–686
Lee AYY, Peterson EA, Wu C (2016) Clinical practice guidelines on cancer-associated thrombosis: a review on scope and methodology. Thromb Res 140(Suppl 1):S119-127
Key NS, Khorana AA, Kuderer NM, et al (2019) Venous thromboembolism prophylaxis and treatment in patients with cancer: ASCO Clinical Practice Guideline Update. J Clin Oncol. JCO1901461
Raskob GE, van Es N, Verhamme P, et al (2017) Edoxaban for the treatment of cancer-associated venous thromboembolism. N Engl J Med
Young A, Marshall A, Thirlwall J et al (2017) anticoagulation therapy in selected cancer patients at risk of recurrence of venous thromboembolism: results of the Select-D Pilot Trial. Blood 130(Suppl 1):625
McBane RD, Wysokinski WE, Le-Rademacher JG, et al. Apixaban and dalteparin in active malignancy-associated venous thromboembolism: The ADAM VTE trial. J Thromb Haemost. October 2019
Li A, Garcia DA, Lyman GH et al (2019) Direct oral anticoagulant (DOAC) versus low-molecular-weight heparin (LMWH) for treatment of cancer associated thrombosis (CAT): a systematic review and meta-analysis. Thromb Res 173:158–163
Mahé I, Chidiac J, Helfer H et al (2016) Factors influencing adherence to clinical guidelines in the management of cancer-associated thrombosis. J Thromb Haemost 14(11):2107–2113
Khorana AA, Dalal M, Lin J et al (2013) Incidence and predictors of venous thromboembolism (VTE) among ambulatory high-risk cancer patients undergoing chemotherapy in the United States. Cancer 119(3):648–655
Mahé I, Sterpu R, Bertoletti L et al (2015) Long-term anticoagulant therapy of patients with venous thromboembolism. What are the practices? PLoS ONE 10(6):e0128741
Wittkowsky AK (2006) Barriers to the long-term use of low-molecular weight heparins for treatment of cancer-associated thrombosis. J Thromb Haemost 4(9):2090–2091
Delate T, Witt DM, Ritzwoller D et al (2012) Outpatient use of low molecular weight heparin monotherapy for first-line treatment of venous thromboembolism in advanced cancer. Oncologist 17(3):419–427
Khorana AA, McCrae KR, Milentijevic D et al (2017) Current practice patterns and patient persistence with anticoagulant treatments for cancer-associated thrombosis. Res Pract Thromb Haemost 1(1):14–22
Cancer Therapy Look-up Tables. National Cancer Institute. https://crn.cancer.gov/resources/codes.html. Accessed 3 Apr 2019
Tamariz L, Harkins T, Nair V (2012) A systematic review of validated methods for identifying venous thromboembolism using administrative and claims data. Pharmacoepidemiol Drug Saf 21(Suppl 1):154–162
Sanfilippo KM, Wang T-F, Gage BF et al (2015) Improving accuracy of International Classification of Diseases codes for venous thromboembolism in administrative data. Thromb Res 135(4):616–620
Heckbert SR, Kooperberg C, Safford MM et al (2004) Comparison of self-report, hospital discharge codes, and adjudication of cardiovascular events in the Women’s Health Initiative. Am J Epidemiol 160(12):1152–1158
Fang MC, Fan D, Sung SH et al (2017) Validity of using inpatient and outpatient administrative codes to identify acute venous thromboembolism: the CVRN VTE study. Med Care 55(12):e137–e143
White RH, Garcia M, Sadeghi B et al (2010) Evaluation of the predictive value of ICD-9-CM coded administrative data for venous thromboembolism in the United States. Thromb Res 126(1):61–67
Go AS, Hylek EM, Chang Y et al (2003) Anticoagulation therapy for stroke prevention in atrial fibrillation: how well do randomized trials translate into clinical practice? JAMA 290(20):2685–2692
Quan H, Sundararajan V, Halfon P et al (2005) Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care 43(11):1130–1139
Desai RJ, Solomon DH, Shadick N et al (2016) Identification of smoking using Medicare data—a validation study of claims-based algorithms. Pharmacoepidemiol Drug Saf 25(4):472–475
Charlson ME, Pompei P, Ales KL et al (1987) A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 40(5):373–383
Hershman DL, Tsui J, Wright JD et al (2015) Household net worth, racial disparities, and hormonal therapy adherence among women with early-stage breast cancer. J Clin Oncol 33(9):1053–1059
Khorana AA, Yannicelli D, McCrae KR et al (2016) Evaluation of US prescription patterns: are treatment guidelines for cancer-associated venous thromboembolism being followed? Thromb Res 145:51–53
Hernandez I, Saba S, Zhang Y (2017) Geographic variation in the use of oral anticoagulation therapy in stroke prevention in atrial fibrillation. Stroke 48(8):2289–2291
Christesen AMS, Vinter N, Mortensen LS et al (2018) Inequality in oral anticoagulation use and clinical outcomes in atrial fibrillation: a Danish nationwide perspective. Eur Heart J Qual Care Clin Outcomes 4(3):189–199
Nathan AS, Geng Z, Dayoub EJ et al (2019) Racial, ethnic, and socioeconomic inequities in the prescription of direct oral anticoagulants in patients with venous thromboembolism in the United States. Circ Cardiovasc Qual Outcomes 12(4):e005600
Noble S, Nelson A, Scott J et al (2020) Patient experience of living with cancer-associated thrombosis in Canada (PELICANADA). Res Pract Thromb Haemost 4(1):154–160. https://doi.org/10.1002/rth2.12274
Author information
Authors and Affiliations
Contributions
JKS, ZW, SLS, and JJG designed the study. JKS, ML, ZW, SLS, JJG, and TB collected and analyzed the data. JKS drafted the manuscript. All authors reviewed and approved the final version.
Corresponding author
Ethics declarations
Conflict of interest
Geoffrey Barnes reports honoraria from Pfizer/BMS, Janssen, Portola, and AMAG Pharmaceuticals, research funding from Blue Cross Blue Shield of Michigan, BMS, Pfizer, and NHLBI. Marc Carrier reports research funding from BMS, Pfizer, Leo Pharma and honoraria from Bayer, BMS, Pfizer, Leo Pharma, Sanofi, and Servier. Suman Sood reports consulting for Bayer. Mengbing Li reports research funding from the Michigan Institute for Data Science (MIDAS). Zhenke Wu reports research funding from the National Cancer Institute of the NIH under award number P30CA046592 through the Cancer Center Support Grant (CCSG), Development Funds from the Rogel Cancer Center, and an investigator award from Michigan Precision Health Initiative, and funds from Michigan Institute for Data Science (MIDAS). All other authors have no conflict of interests to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Schaefer, J.K., Li, M., Wu, Z. et al. Clinical and sociodemographic factors associated with anticoagulant use for cancer associated venous thromboembolism. J Thromb Thrombolysis 52, 214–223 (2021). https://doi.org/10.1007/s11239-021-02392-9
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11239-021-02392-9