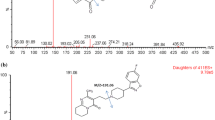
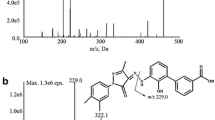
Abstract
Although DOACs do not require regular measurements of their blood concentrations, clinical situations may require an assessment of their concentration. Among the factor Xa inhibitors, edoxaban is the only compound for which some metabolites (e.g. edoxaban-M4) are reported to be pharmacologically active. Therefore, their contribution could interfere with assays used for the estimation of edoxaban concentration. In addition, drug interactions may alter the metabolite/parent compound ratio making the sole estimation of edoxaban concentration, a poor assessment of the overall anticoagulation. To develop a validated UHPLC–MS/MS method to quantify simultaneously edoxaban and its more relevant M4-metabolite in human plasma. Electrospray ionization and chromatographic separation were optimized for the simultaneous dosage of edoxaban and edoxaban-M4. The method was validated according to regulatory guidelines for bioanalytical method validation. The total run time was 6 min. The method was validated for calibration curves, precision, accuracy, carry-over, selectivity, matrix effect and short-time stability. This method permits quantification of edoxaban and edoxaban-M4 providing complementary information about the inhibitory effect of this active metabolite in chronometric or chromogenic assays. Although patients treated with edoxaban exhibits usually low concentrations of active metabolites, the measurement of edoxaban-M4 is interesting; especially in case of drug interactions. Indeed, concomitant prescriptions of edoxaban and carbamazepine or rifampicin is frequent and may lead to disturbance of the estimations of edoxaban concentration by chromogenic anti-Xa assays. Therefore, patients are at risk of having inadequate control of anticoagulation supporting the need of measuring the most representative edoxaban metabolite concomitantly to the parent compound.



Similar content being viewed by others
Abbreviations
- %Dev:
-
Percentage of relative standard deviation
- CV:
-
Coefficient of variation
- DMSO:
-
Dimethyl sulfoxide
- DOACs:
-
Direct oral anticoagulants
- EMA:
-
European Medicines Agency
- ESI:
-
Electro spray ionization
- ESI + :
-
Electro spray on positive ionization mode
- FDA:
-
Food and Drug Administration
- ICH:
-
International Council of Harmonization
- IS:
-
Internal standard
- LC:
-
Liquid chromatography
- LLOQ:
-
Lower limit of quantification
- ME:
-
Matrix effect
- MRM:
-
Multiple reaction monitoring
- MS:
-
Mass spectrometry
- NPP:
-
Normal pooled plasma
- PBS:
-
Phosphate buffer saline
- PPP:
-
Platelet poor plasma
- QCs:
-
Quality controls
- UHPLC–MS/MS:
-
Ultra-high-performance liquid chromatography coupled with mass spectrometry
- ULOQ:
-
Upper limit of quantification
- VKAs:
-
Vitamin K antagonists
- VTE:
-
Venous thromboembolisms
References
Siriez R et al (2019) Development of new methodologies for the chromogenic estimation of betrixaban concentrations in plasma. Int J Lab Hematol. https://doi.org/10.1111/ijlh.12963
Siriez R et al (2018) Betrixaban: impact on routine and specific coagulation assays-a practical laboratory guide. Thromb Haemost 118(7):1203–1214
Douxfils J et al (2018) Laboratory testing in patients treated with direct oral anticoagulants: A practical guide for clinicians. J Thromb Haemost 16(2):209–219
Douxfils J, Gosselin RC (2017) Laboratory assessment of direct oral anticoagulants. Semin Thromb Hemost 43(3):277–290
Douxfils J et al (2016) Edoxaban: impact on routine and specific coagulation assays A practical laboratory guide. Thromb Haemost 115(2):368–381
Douxfils J et al (2013) Impact of apixaban on routine and specific coagulation assays: a practical laboratory guide. Thromb Haemost 110(2):283–294
Douxfils J et al (2012) Assessment of the impact of rivaroxaban on coagulation assays: laboratory recommendations for the monitoring of rivaroxaban and review of the literature. Thromb Res 130(6):956–966
Douxfils J et al (2012) Impact of dabigatran on a large panel of routine or specific coagulation assays Laboratory recommendations for monitoring of dabigatran etexilate. Thromb Haemost 107(5):985–997
Skeppholm M et al (2014) On the monitoring of dabigatran treatment in "real life" patients with atrial fibrillation. Thromb Res 134(4):783–789
Parasrampuria DA, Truitt KE (2016) Pharmacokinetics and pharmacodynamics of edoxaban, a non-vitamin k antagonist oral anticoagulant that inhibits clotting factor xa. Clin Pharmacokinet 55(6):641–655
Food and Drug Administration (2014) Clinical pharmacology and biopharmaceutics review(s)
Parasrampuria DA et al (2016) Edoxaban drug–drug interactions with ketoconazole, erythromycin, and cyclosporine. Br J Clin Pharmacol 82(6):1591–1600
Food and Drug Administration (2018) Bioanalytical method validation: guidance for industry. https://www.fda.gov/downloads/drugs/guidances/ucm070107.pdf. Accessed 6 Feb 2019
He L et al (2017) Determination of edoxaban equivalent concentrations in human plasma by an automated anti-factor Xa chromogenic assay. Thromb Res 155:121–127
EMA, ICH Topic Q2 (R1) (1995) Validation of analytical procedures: text and methodology—NOTE FOR GUIDANCE ON VALIDATION OF ANALYTICAL PROCEDURES: TEXT AND METHODOLOGY (CPMP/ICH/381/95)
Rohde G (2008) Determination of rivaroxaban—a novel, oral, direct factor Xa inhibitor—in human plasma by high-performance liquid chromatography-tandem mass spectrometry. J Chromatogr B 872(1–2):43–50
Lagoutte-Renosi J et al (2018) A simple and fast HPLC–MS/MS method for simultaneous determination of direct oral anticoagulants apixaban, dabigatran, rivaroxaban in human plasma. J Chromatogr B 1100–1101:43–49
Foerster KI et al (2018) Simultaneous quantification of direct oral anticoagulants currently used in anticoagulation therapy. J Pharm Biomed Anal 148:238–244
Mendell J et al (2015) The effect of rifampin on the pharmacokinetics of edoxaban in healthy adults. Clin Drug Investig. https://doi.org/10.1007%2Fs40261-015-0298-2
Ruff CT et al (2015) Association between edoxaban dose, concentration, anti-factor Xa activity, and outcomes: an analysis of data from the randomised, double-blind ENGAGE AF-TIMI 48 trial. Lancet 385(9984):2288–2295
Verhamme P et al (2016) Dose reduction of edoxaban preserves efficacy and safety for the treatment of venous thromboembolism An analysis of the randomised, double-blind HOKUSAI VTE trial. Thromb Haemost 116(4):747–753
Weitz JI et al (2010) Randomised, parallel-group, multicentre, multinational phase 2 study comparing edoxaban, an oral factor Xa inhibitor, with warfarin for stroke prevention in patients with atrial fibrillation. Thromb Haemost 104(3):633–641
Levy JH et al (2016) When and how to use antidotes for the reversal of direct oral anticoagulants: guidance from the SSC of the ISTH. J Thromb Haemost 14(3):623–627
CBIP (2018) Centre Belge d’Information Pharmacothérapeutique - Bon usage des médicaments - Interactions des médicaments
Vazquez SR (2018) Drug-drug interactions in an era of multiple anticoagulants: a focus on clinically relevant drug interactions. Hematol Am Soc Hematol Educ Program 2018(1):339–347
Wang JZ et al (2017) Incidence and management of seizures after ischemic stroke: systematic review and meta-analysis. Neurology 89(12):1220–1228
Jabareen A et al (2018) Treatment with antiepileptic drugs in patients with stroke. A change in clinical practice may be required. J Neurol Sci 395:4–7
Galgani A et al (2018) Pharmacokinetic interactions of clinical interest between direct oral anticoagulants and antiepileptic drugs. Front Neurol 9:1067
Di Gennaro L et al (2019) Carbamazepine interaction with direct oral anticoagulants: help from the laboratory for the personalized management of oral anticoagulant therapy. J Thromb Thrombolysis. https://doi.org/10.1007/s11239-019-01866-1
Stollberger C, Finsterer J (2016) Interactions between non-vitamin K oral anticoagulants and antiepileptic drugs. Epilepsy Res 126:98–101
European Medicines Agency (2015) Lixiana: EMEA/H/C/002629/0000, 25 Apr 2015. https://www.ema.europa.eu/en/documents/assessment-report/lixiana-epar-public-assessment-report_en.pdf. Accessed 23 Oct 2019
Food and Drug Administration (2015) Savaysa: clinical pharmacology and biopharmaceutics review(s). 2015. Accessed 12 Jun 2015
Acknowledgements
The authors would like to acknowledge Christelle Vancraeynest, Vincent Maloteau, Lionel Pochet and Jonathan Evrard for their contribution to this work. The authors would also like to thank the NAB-X biobank from the University of Namur (UNamur) for the management and storage of plasma samples.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The other authors have no conflicts of interest to disclose.
Ethical approval
Among the authors, J. Douxfils is CEO and founder of QUALIblood s.a. and reports personal fees from Portola Pharmaceuticals, Stago, Roche, Roche Diagnostics and Daiichi-Sankyo, outside the submitted work.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.







Rights and permissions
About this article
Cite this article
Siriez, R., Alpan, L., Elasaad, K. et al. Importance of measuring pharmacologically active metabolites of edoxaban: development and validation of an ultra-high-performance liquid chromatography coupled with a tandem mass spectrometry method. J Thromb Thrombolysis 49, 395–403 (2020). https://doi.org/10.1007/s11239-019-02030-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11239-019-02030-5