Abstract
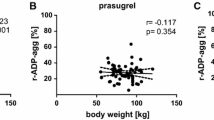
Recent reports have suggested that aspirin effect might be influenced by bodyweight, with decreased efficacy in heavier individuals. We investigated the influence of bodyweight on aspirin pharmacodynamics in two independent datasets of patients taking non-enteric coated aspirin 100 mg QD for coronary artery disease (CAD). In the first dataset, 368 patients had their platelet aggregation assessed using VerifyNow Aspirin and measured in Aspirin Reaction Units (ARU). In the second dataset, 70 patients had serum thromboxane B2 (TXB2) dosage assessed by an ELISA assay and measured in pg/mL. Platelet aggregation was independently associated with bodyweight, with 8.41 (95% CI 1.86–14.97; adjusted p-value = 0.012) increase in ARU for every 10 kg. Furthermore, the rate of non-response to aspirin (defined as ARU ≥ 550) was significantly associated with increased bodyweight (adjusted p-value = 0.007), with OR = 1.23 (95% CI 1.06–1.42) for every 10 kg. Similar results were found considering body mass index (in kg/m2), with 15.5 (95% CI 5.0 to 25.9; adjusted p-value = 0.004) increase in ARU for every 10 kg and non-response OR = 1.43 (95% CI 1.13 to 1.81, adjusted p-value = 0.003) for every 5 kg/m2. Moreover, serum TXB2 was higher in patients weighting more than 70 kg (222.6 ± 62.9 versus 194.9 ± 61.9 pg/mL; adjusted p-value = 0.018). In two different datasets of patients with CAD on non-enteric coated aspirin 100 mg QD, increased bodyweight was independently associated with impaired response to aspirin.


Similar content being viewed by others
References
Baigent C, Blackwell L, Collins R, Antithrombotic Trialists’ Collaboration et al (2009) Aspirin in the primary and secondary prevention of vascular disease: collaborative meta-analysis of individual participant data from randomised trials. Lancet 373:1849–1860
Antithrombotic Trialists’ Collaboration (2002) Collaborative meta-analysis of randomised trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke in high risk patients. BMJ 324:71–86
Lewis HD Jr, Davis JW, Archibald DG et al (1983) Protective effects of aspirin against acute myocardial infarction and death in men with unstable angina. Results of a Veterans Administration Cooperative Study. N Engl J Med 309:396–403
ISIS-2 (Second International Study of Infarct Survival) Collaborative Group (1988) Randomised trial of intravenous streptokinase, oral aspirin, both, or neither among 17,187 cases of suspected acute myocardial infarction: ISIS-2. Lancet 2:349–360
Levine GN, Bates ER, Bittl JA et al (2016) 2016 ACC/AHA Guideline Focused Update on Duration of Dual Antiplatelet Therapy in Patients With Coronary Artery Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 134:e123–e155
Valgimigli M, Bueno H, Byrne RA et al (2018) 2017 ESC focused update on dual antiplatelet therapy in coronary artery disease developed in collaboration with EACTS: The Task Force for dual antiplatelet therapy in coronary artery disease of the European Society of Cardiology (ESC) and of the European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J 39:213–260
Krasopoulos G, Brister SJ, Beattie WS, Buchanan MR (2008) Aspirin “resistance” and risk of cardiovascular morbidity: systematic review and meta-analysis. BMJ 336:195–198
Dupont AG, Gabriel DA, Cohen MG (2009) Antiplatelet therapies and the role of antiplatelet resistance in acute coronary syndrome. Thromb Res 124:6–13
Rothwell PM, Cook NR, Gaziano JM et al (2018) Effects of aspirin on risks of vascular events and cancer according to bodyweight and dose: analysis of individual patient data from randomised trials. Lancet 392:387–399
Mehta SR, Bassand JP, Chrolavicius S et al (2010) Dose comparisons of clopidogrel and aspirin in acute coronary syndromes. N Engl J Med 363:930–942
Maree AO, Curtin RJ, Dooley M et al (2005) Platelet response to low-dose enteric-coated aspirin in patients with stable cardiovascular disease. J Am Coll Cardiol 46:1258–1263
Peace A, McCall M, Tedesco T et al (2010) The role of weight and enteric coating on aspirin response in cardiovascular patients. J Thromb Haemost 8:2323–2325
Chen WH, Lee PY, Ng W, Tse HF, Lau CP (2004) Aspirin resistance is associated with a high incidence of myonecrosis after non-urgent percutaneous coronary intervention despite clopidogrel pretreatment. J Am Coll Cardiol 43:1122–1126
VerifyNow Aspirin Test [package insert]. Accriva Diagnostics, San Diego, CA. http://www.accriva.com/uploads/literature/vn1011en-web_00.pdf. Accessed 8 Jan 2019
Gum PA, Kottke-Marchant K, Poggio ED et al (2001) Profile and prevalence of aspirin resistance in patients with cardiovascular disease. Am J Cardiol 88:230–235
Pulcinelli FM, Biasucci LM, Riondino S et al (2009) COX-1 sensitivity and thromboxane A2 production in type 1 and type 2 diabetic patients under chronic aspirin treatment. Eur Heart J 30:1279–1286
Larsen SB, Grove EL, Neergaard-Petersen S, Würtz M, Hvas AM, Kristensen SD (2015) Determinants of reduced antiplatelet effect of aspirin in patients with stable coronary artery disease. PLoS ONE 10(5):e0126767
Stone GW, Witzenbichler B, Weisz G et al (2013) Platelet reactivity and clinical outcomes after coronary artery implantation of drug-eluting stents (ADAPT-DES): a prospective multicentre registry study. Lancet 382:614–623
Eikelboom JW, Hirsh J, Weitz JI, Johnston M, Yi Q, Yusuf S (2002) Aspirin-resistant thromboxane biosynthesis and the risk of myocardial infarction, stroke, or cardiovascular death in patients at high risk for cardiovascular events. Circulation 105:1650–1655
Hao Y, Liu J, Liu J et al. on behalf of the CCC-ACS Investigators. (2019) Sex differences in in-hospital management and outcomes of patients with acute coronary syndrome: findings from the improving care for cardiovascular disease in China (CCC) project. Circulation [epub ahead of print]
Cox D, Maree AO, Dooley M, Conroy R, Byrne MF, Fitzgerald DJ (2006) Effect of enteric coating on antiplatelet activity of low-dose aspirin in healthy volunteers. Stroke 37:2153–2158
Bhatt DL, Grosser T, Dong JF et al (2017) Enteric coating and aspirin nonresponsiveness in patients with Type 2 diabetes mellitus. J Am Coll Cardiol 69:603–612
Voora D, Ortel TL, Lucas JE, Chi JT, Becker RC, Ginsburg GS (2012) Time-dependent changes in non-COX-1-dependent platelet function with daily aspirin therapy. J Thromb Thrombolysis 33:246–257
Sankaralingam S, Kim RB, Padwal RS (2015) The impact of obesity on the pharmacology of medications used for cardiovascular risk factor control. Can J Cardiol 31:167–176
Rocca B, Petrucci G (2012) Variability in the responsiveness to low-dose aspirin: pharmacological and disease-related mechanisms. Thrombosis 2012:1–11
Vilahur G, Ben-Aicha S, Badimon L (2017) New insights into the role of adipose tissue in thrombosis. Cardiovasc Res 113:1046–1054
Rocca B, Fox KAA, Ajjan RA et al (2018) Antithrombotic therapy and body mass: an expert position paper of the ESC Working Group on Thrombosis. Eur Heart J 39:1672–1686
De Luca L, Temporelli PL, Riccio C et al (2019) Clinical outcomes, pharmacological treatment, and quality of life of patients with stable coronary artery diseases managed by cardiologists: 1-year results of the START study. Eur Heart J 0:1–9
Funding
This study was supported by grants from Sao Paulo Research Foundation (FAPESP) and Brazilian National Council of Research (CNPQ). This study was financed in part by the “Coordenação de Aperfeiçoamento de Pessoal de Nível Superior”—Brazil (CAPES)-Finance Code 001. The funding sources had no role in study design, decision for publication or data analyses.
Author information
Authors and Affiliations
Contributions
RHMF contributed to study design, data collection, statistical analysis, data interpretation, and drafting of the manuscript, RPG contributed to data interpretation and critical review of the manuscript, TFD contributed to data collection, data interpretation, and critical review of the manuscript, FBB contributed to data collection, data interpretation, and critical review of the manuscript, CJDGB contributed to data collection, data interpretation, and critical review of the manuscript, PRRG contributed to data interpretation and critical review of the manuscript, AF contributed to data collection, data interpretation, and critical review of the manuscript, FRM contributed to data collection, data interpretation, and critical review of the manuscript, CAKN contributed to data collection, data interpretation, and critical review of the manuscript, MASF contributed to data collection, data interpretation, and critical review of the manuscript, AGF contributed to data collection, data interpretation, and critical review of the manuscript, RS contributed to data collection, statistical analysis and critical review of the manuscript, LMB contributed to data interpretation and critical review of the manuscript, JCN contributed to study design, data interpretation, and critical review of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
R.H.M. Furtado: Honoraria: AstraZeneca; Research Grant: AstraZeneca, DalCor, Boehinger, Pfizer, Bayer, Sanofi. R.P. Giugliano: Research Grant: Amgen, Merck. Honoraria; Significant; Amgen, Daiichi Sankyo, Merck. Consultant/Advisory Board; Significant; Amarin, Amgen, Boehringer- Ingelheim, Bristol-Myers-Squibb, CVS Caremark, Daiichi Sankyo, GlaxoSmithKline, Lexicon, Merck, Portola, Pfizer. T.F. Dalcoquio: Travel grant: Bayer; Research Grant: AstraZeneca, DalCor P.R.R. Genestreti: Advisory board: NovoNordisk, Sanofi, AstraZeneca; Speaker board: NovoNordisk, Sanofi, AstraZeneca, Boeringher-Ingelhbeim, Eli-Lilly, L.M. Baracioli: Research Grant: AstraZeneca, DalCor J.C. Nicolau: Research Grant: Sao Paulo Research Foundation, Amgen Inc., Bayer Healthcare Pharmaceuticals, Bristol-Myers Squibb Company, DalCor, Janssen Pharmaceuticals Inc, Sanofi-Aventis, Astra Zeneca, Boehringer Ingelheim, Novartis, Pfizer Inc. Consultant/Advisory Board; Significant; Sanofi-Aventis. All other co-authors have nothing to disclose.
Ethical approval
Patients with stable CAD were enrolled from two randomized clinical trials (NCT02389582 and NCT 01896557) and one case-control study conducted at our institution. These three studies were approved by our institutional review board and all patients signed informed consent form (ICF) before obtaining blood test for assessment of platelet aggregation or serum TXB2 dosage. In the case of patients with ACS, platelet aggregation was obtained in a routine basis at the CCU discharge and, because of that, ICF was waived according to local regulations. All data from this report were analyzed by two independent authors (RHMF and RS) using de-identified patient information.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Furtado, R.H.M., Giugliano, R.P., Dalcoquio, T.F. et al. Increased bodyweight and inadequate response to aspirin in individuals with coronary artery disease. J Thromb Thrombolysis 48, 217–224 (2019). https://doi.org/10.1007/s11239-019-01830-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11239-019-01830-z