Abstract
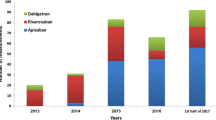
Direct oral anticoagulants (DOACs) are commonly administered at a level that is lower than that recommended by dose reduction criteria. This raises concern regarding the adequacy of anticoagulation achieved. To evaluate the relationship between inappropriate dosing and DOAC levels. Medical records of atrial fibrillation patients who underwent DOAC level testing during 2013–2017 were reviewed. The primary outcomes were drug levels under and above the expected steady-state range, and in the lowest and highest quartiles. Of 143 patients who underwent DOAC measurements, only 87 (60.8%) received the appropriate dose. Levels under the expected range and in the lowest quartile were found in 11.9% and 15.0% of patients treated with appropriate dosing compared to 21% and 41.5% of patients treated with inappropriately low dose. DOAC levels were above the expected range and in the highest quartile in 23.8% and 32.5% of patients treated with the appropriate dose compared to 7.1% and 9.4% treated with inappropriately low dose. In multivariate analysis, the administration of an appropriate DOAC dose was associated with a lower rate of DOAC in the lowest level (adjusted odds ratio [95% CI] 0.30 (0.12, 0.76), P = 0.011). On the other hand, appropriate dose was associated with drug levels in the highest quartile (odds ratio [95% CI] 3.77 (0.12, 0.76), P = 0.011). Treatment with inappropriately low DOAC dosing compared to appropriate dose is associated with lower DOAC levels. However, among those treated with appropriate dosing, a higher proportion had high DOAC levels above the expected range.
Similar content being viewed by others
References
Camm a J, Kirchhof P, Lip GYH et al (2010) Guidelines for the management of atrial fibrillation: the Task Force for the Management of Atrial Fibrillation of the European Society of Cardiology (ESC). Europace 12:1360–1420. https://doi.org/10.1093/europace/euq350
Steffel J, Verhamme P, Potpara TS et al (2018) The 2018 European Heart Rhythm Association Practical Guide on the use of non-vitamin K antagonist oral anticoagulants in patients with atrial fibrillation. Eur Heart J 39(April):1330–1393. https://doi.org/10.1093/eurheartj/ehy136
Kirchhof P, Uk C, Uk DK et al (2016) 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS The Task Force for the management of atrial fibrillation of the European Society of Cardiology (ESC) Developed with the special contribution of the Europ. Eur Heart J 37(38):7–90. https://doi.org/10.1093/eurheartj/ehw210
Granger CB, Alexander JH, McMurray JJ et al (2011) Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med 365(11):981–992
Patel MR, Mahaffey KW, Garg J et al (2010) Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med 365(10):609–619. https://doi.org/10.1056/NEJMoa1109071
Connolly SJ, Ezekowitz MD, Yusuf S et al (2009) Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med 361(12):1139–1151
Ruiz Ortiz M, Muñiz JRMP et al. Inappropriate doses of direct oral anticoagulants in real-world clinical practice: prevalence and associated factors. A subanalysis of the FANTASIIA Registry. Europace. 2017;(May):1–7. https://doi.org/10.1093/europace/eux316
Shinoda N, Mori M, Tamura S, Korosue K, Kose S, Kohmura E (2018) Risk of recurrent ischemic stroke with unintended low-dose oral anticoagulant therapy and optimal timing of review. J Stroke Cerebrovasc Dis 27(6):1546–1551. https://doi.org/10.1016/j.jstrokecerebrovasdis.2018.01.002
Basaran O, Basaran NF, Cekic EG et al (2014) PRescriptiOn PattERns of oral anticoagulants in nonvalvular atrial fibrillation (PROPER study). Ann Pharmacother 48(10):1258–1268. https://doi.org/10.1177/1076029615614395
Howard M, Lipshutz A, Roess B et al (2017) Identification of risk factors for inappropriate and suboptimal initiation of direct oral anticoagulants. J Thromb Thrombolysis 43(2):149–156
Drug Development and Drug Interactions (2017) Table of substrates, inhibitors and inducers. https://www.fda.gov/Drugs/DevelopmentApprovalProcess/DevelopmentResources/DrugInteractionsLabeling/ucm093664.htm#table3-2
Eikelboom JW, Quinlan DJ, Hirsh J, Connolly SJ, Weitz JI (2017) Laboratory monitoring of non–vitamin k antagonist oral anticoagulant use in patients with atrial fibrillation—a review. JAMA Cardiol 2(5):566–574. https://doi.org/10.1001/jamacardio.2017.0364
Ryn J, Van Stangier J, Haertter S, Liesenfeld K, Wienen W, Feuring M (2010) Dabigatran etexilate—a novel, reversible, oral direct thrombin inhibitor: interpretation of coagulation assays and reversal of anticoagulant activity. Thromb Haemost 2010; 103(6):1116–1127. https://doi.org/10.1160/TH09-11-0758
Testa S, Paoletti O, Legnani C, Antonucci E (2018) Low drug levels and thrombotic complications in high-risk atrial fibrillation patients treated with direct oral anticoagulants. J Thromb Haemost 16(5):842–848. https://doi.org/10.1111/jth.14001
Louw S, Saragas NP, Ferrao PN, Chirwa TF (2016) BFJ. Correlation between rivaroxaban (Xarelto) plasma activity, patient clinical variables and outcomes in a South African centre. S Afr Med J 106(10):1017–1020. https://doi.org/10.1111/jth.14001
Center for Drug Evaluation and Research (2014) Cardiovascular And Renal Drugs Advisory Committee Briefing Document Savaysa™, Edoxaban. http://www.fda.gov/downloads/advisorycommittees/committeesmeetingmaterials/drugs/cardiovascularandrenaldrugsadvisorycommittee/ucm420705.pdf
Center for Drug Evaluation and Research Application Number 202439Orig1s000, Medical Review, Rivaroxaban (2011). http://www.accessdata.fda.gov/drugsatfda_docs/nda/2011/202439Orig1s000MedR.pdf
Center for Drug Evaluation and Research Application Number:22–512, Medical Review, Dabigatran (2010). http://www.accessdata.fda.gov/drugsatfda_docs/nda/2010/022512Orig1s000MedR.pdf
Acknowledgements
We thank Ms. Cindy Cohen for her editorial assistance.
Funding
No external funding was used in this conduct of this study.
Author information
Authors and Affiliations
Contributions
All authors-study concept, design, review and approval of the final manuscript. AR, NZ, BHR, AP, HDD, IM and YK reviewed the literature and wrote the paper. Authors AR, YK and NZ collected the data.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Informed consent
IRB approval waiving informed consent was obtained for this retrospective study.
Statement of human and animal rights
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. This study was approved by the local institutional review board of Hadassah Medical Center Helsinki Committee (IRB Approval Number No. HMO 0126-17).
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Hirsh Raccah, B., Rottenstreich, A., Zacks, N. et al. Appropriateness of direct oral anticoagulant dosing and its relation to drug levels in atrial fibrillation patients. J Thromb Thrombolysis 47, 550–557 (2019). https://doi.org/10.1007/s11239-019-01815-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11239-019-01815-y